��Ŀ����
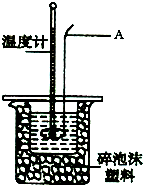 ijʵ��С����0.50mol?L-1NaOH��Һ��0.50mol?L-1��1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol?L-1NaOH��Һ��0.50mol?L-1��1������Һ�����к��ȵIJⶨ��������0.50mol?L-1������Һ
��1��������250mL������Һ����������Ͳ��ȡ�ܶ�Ϊ1.84g?cm-3����������Ϊ98%��Ũ����
�ⶨϡ�����ϡ����������Һ�к��ȵ�ʵ��װ������ͼ��ʾ��
��2������A������Ϊ
��3��װ��������ĭ���ϵ�������
��4��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ�����к���Ϊ57.3kJ?mol-1��
��5��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���
�ٱ��е��¶Ȳ�ƽ��ֵΪ
�ڽ�����Ϊ0.50mol?L-1 NaOH��Һ��0.50mol?L-1������Һ���ܶȶ���1g?cm-3���кͺ�������Һ�ı�����c=4.18J?��g?�棩-1�����к��ȡ�H=
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳt2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.6 | 26.6 | 26.6 | 29.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.2 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c��һ����NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�
���㣺�к��ȵIJⶨ
ר�⣺��ѧ��Ӧ�е������仯
������I����1��c=n/V���=m/MV=w��v/M��0.25L=98%��1.84g/cm3��V/98g/mol��0.25L=0.5mol/L��v=6.8ml��
II����2��Ϊ��ֵĽ��裬�û��β��������������3������ĭ�������DZ��¡����ȡ�����������ʧ��
��4��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������Ӧ����1molҺ̬ˮ��
��5���ٸ��ݱ�����������²�ƽ��ֵ��
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m?c?��T=80g��4.18J/��g?�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-1.3376KJ/0.025mol=-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС��
II����2��Ϊ��ֵĽ��裬�û��β��������������3������ĭ�������DZ��¡����ȡ�����������ʧ��
��4��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������Ӧ����1molҺ̬ˮ��
��5���ٸ��ݱ�����������²�ƽ��ֵ��
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m?c?��T=80g��4.18J/��g?�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-1.3376KJ/0.025mol=-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС��
���
�⣺I����1��c=n/V���=m/MV=w��v/M��0.25L=98%��1.84g/cm3��V/98g/mol��0.25L=0.5mol/L����v=6.8mL���ʴ�Ϊ��6.8��
II����2��Ϊ��ֵĽ��裬�û��β�������������ʴ�Ϊ�����β�������
��3������ĭ�������DZ��¡����ȡ�����������ʧ���ʴ�Ϊ�����¡����ȡ�����������ʧ��
��4��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������Ӧ����1molҺ̬ˮ���Ȼ�ѧ����ʽΪ
H2SO4��aq��+NaOH��aq��=
Na2SO4��aq��+H2O��l����H=-57.3kJ/mol���ʴ�Ϊ��
H2SO4��aq��+NaOH��aq��=
Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��5���ٸ��ݱ�����������²�ƽ��ֵΪ
��=4.0�棬�ʴ�Ϊ��4.0��
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m?c?��T=80g��4.18J/��g?�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-1.3376KJ/0.025mol=-53.5kJ/mol���ʴ�Ϊ��-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����aѡ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b��ѡ��
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��ɱ�֤ʵ��ɹ�����c��ѡ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����ѡ��
�ʴ�Ϊ��ad��
II����2��Ϊ��ֵĽ��裬�û��β�������������ʴ�Ϊ�����β�������
��3������ĭ�������DZ��¡����ȡ�����������ʧ���ʴ�Ϊ�����¡����ȡ�����������ʧ��
��4��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������Ӧ����1molҺ̬ˮ���Ȼ�ѧ����ʽΪ
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
��5���ٸ��ݱ�����������²�ƽ��ֵΪ
| 3.5+4+3.9+4.1 |
| 4 |
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m?c?��T=80g��4.18J/��g?�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-1.3376KJ/0.025mol=-53.5kJ/mol���ʴ�Ϊ��-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����aѡ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b��ѡ��
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��ɱ�֤ʵ��ɹ�����c��ѡ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����ѡ��
�ʴ�Ϊ��ad��
���������⿼���Ȼ�ѧ����ʽ�Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷȴ�ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�������Լ��ⶨ��Ӧ�ȵ��������⣮

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ
���������ڿ����в����ȶ����ڵ��ǣ�������
| A��Fe��OH��2 |
| B��Fe��OH��3 |
| C��K3Fe��CN��6 |
| D��Cu��NH3��4SO4 |
��ͬ�¶ȡ���ͬŨ���µ����ֵ������Һ����pH��С�����˳����ͼ��ʾ��ͼ�У��٢ڢ۴��������ʿ��ֱܷ�Ϊ��������
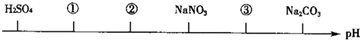
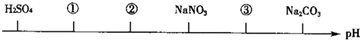
| A��NH4Cl����NH4��2SO4��CH3COONa |
| B����NH4��2SO4��NH4Cl��CH3COONa |
| C����NH4��2SO4��NH4Cl��NaOH |
| D��CH3COOH��NH4Cl����NH4��2SO4 |

 ��0.01mol��AlCl3��Һ�еμ�2mol/L��NaOH��Һʱ�����õij������������NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ��
��0.01mol��AlCl3��Һ�еμ�2mol/L��NaOH��Һʱ�����õij������������NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ��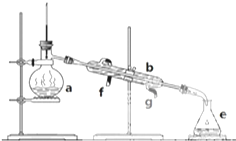 �����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�����ͼΪʵ��װ�ã�
�����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�����ͼΪʵ��װ�ã�