��Ŀ����
��Na+Ũ��Ϊ0.5mol?L-1��ij������Һ�У������ܺ����±��е����������ӣ�
��ȡ����Һ100mL��������ʵ�飨����������ڱ�״���²ⶨ������ش��������⣮
��1��ʵ�����ȷ��һ�������ڵ������� ��
��2��ʵ��������������ᷴӦ��������������ӣ�����������Ҳ�ܷ�Ӧ��д�����������ᷴӦ�����ӷ���ʽΪ ��
��3��ͨ��ʵ���ͱ�Ҫ���㣬��д�±��������ӵ�Ũ�ȣ��ܼ�����ģ���д��������һ�������ڵ������0��������ȷ���Ƿ���ڵ��������������
��4���ж�K+�Ƿ���ڣ�������������СŨ�ȣ���������˵�����ɣ� ��
| ������ | K+��Ag+��Mg2+��Ba2+ |
| ������ | NO3-��CO32-��SiO32-��SO42- |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�� ��������ϡ���� | ������ɫ�������ų���״����0.56 L���� |
| �� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4 g |
| �� | ������Һ�еμ�BaCl2��Һ | ���������� |
��2��ʵ��������������ᷴӦ��������������ӣ�����������Ҳ�ܷ�Ӧ��д�����������ᷴӦ�����ӷ���ʽΪ
��3��ͨ��ʵ���ͱ�Ҫ���㣬��д�±��������ӵ�Ũ�ȣ��ܼ�����ģ���д��������һ�������ڵ������0��������ȷ���Ƿ���ڵ��������������
| ������ | NO3- | CO32-�� | SiO32- | SO42- |
| c/mol?L-1 |
���㣺�������ӵļ��鷽��
ר�⣺
������������֪��ҺΪ������Һ�������Һ�к��е����ӱ����ܴ������森��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ
=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ
=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
| ||
| 0.1L |
| ||
| 0.1L |
���
�⣺������֪��ҺΪ������Һ�������Һ�к��е����ӱ����ܴ������森��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ
=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����SiO32-��Ũ��Ϊ
=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
��1����ʵ����֪����������ϡ�������ɰ�ɫ�������ڱ�״���·ų�0.56L���壬�����Һ��һ������CO32-��SiO32-����һ��û��Ag+��Mg2+��Ba2+��
�ʴ�Ϊ��Ag+��Mg2+��Ba2+��
��2��ʵ��������������ᷴӦ���������������ΪCO32-�����������ᷴӦ����HCO3-�������������ᷴӦ���ɶ�����̼��ˮ���ʷ�Ӧ���ӷ���ʽΪCO32-+H+=HCO3-��
�ʴ�Ϊ��CO32-+H+=HCO3-��
��3��ͨ���������������֪��
�ʴ�Ϊ��
��4�����ݵ���غ�2c��CO32-+��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������K+������Ũ������Ϊ0.8mol/L��
�ʴ�Ϊ�����ڣ���Ũ������Ϊ0.8mol/L��
| ||
| 0.1L |
| ||
| 0.1L |
��1����ʵ����֪����������ϡ�������ɰ�ɫ�������ڱ�״���·ų�0.56L���壬�����Һ��һ������CO32-��SiO32-����һ��û��Ag+��Mg2+��Ba2+��
�ʴ�Ϊ��Ag+��Mg2+��Ba2+��
��2��ʵ��������������ᷴӦ���������������ΪCO32-�����������ᷴӦ����HCO3-�������������ᷴӦ���ɶ�����̼��ˮ���ʷ�Ӧ���ӷ���ʽΪCO32-+H+=HCO3-��
�ʴ�Ϊ��CO32-+H+=HCO3-��
��3��ͨ���������������֪��
| ������ | NO3- | CO32-�� | SiO32- | SO42- |
| c/mol?L-1 | �� | 0.25 | 0.4 | 0 |
| ������ | NO3- | CO32-�� | SiO32- | SO42- |
| c/mol?L-1 | �� | 0.25 | 0.4 | 0 |
�ʴ�Ϊ�����ڣ���Ũ������Ϊ0.8mol/L��
���������⿼�����ӹ���ȣ��Ѷ��еȣ�ע���������ӷ�Ӧ�����ݵ���غ��ж�K+�Ƿ���ڣ��DZ�����ѵ㡢�״��㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
����������������������������ж��У�����ȷ���ǣ�������
| A��������Է������������ݣ��Ʋ��Ҵ��ͱ���е����Ըߵ� |
| B�������ܽ�����ݣ�ѡ������ȡ�ķ����������ᴿ |
| C�����ݷе����ݣ��ж��÷���ķ�����ʯ���л�ȡ���ͺ�ú�͵� |
| D�����ݱ��������ӳɷ�Ӧ�ķ�Ӧ������������ϩ���ļӳɷ�Ӧ�Աȣ��о�����ѧ�������� |

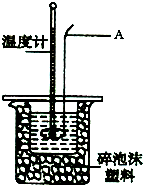 ijʵ��С����0.50mol?L-1NaOH��Һ��0.50mol?L-1��1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol?L-1NaOH��Һ��0.50mol?L-1��1������Һ�����к��ȵIJⶨ��


