��Ŀ����
9��F��һ�����иߵ��ԡ��ͺ��Ժ���ĥ�����ܣ�X��һ�ֺϳ���֬�����ߵĹ�ҵ�ϳ�·����ͼ��ʾ��
��֪��
��

��
 ��-R��-R�䡢-R���ʾ������ͬ����ܲ�ͬ��ԭ�ӻ�ԭ���ţ�
��-R��-R�䡢-R���ʾ������ͬ����ܲ�ͬ��ԭ�ӻ�ԭ���ţ���1���ٷ�Ӧ������Ϊ�ӳɷ�Ӧ��
��2��A�����к˴Ź����������շ�ֻ��1�飬��A�Ľṹ��ʽΪCH3COCH3��
��3��A��C�Ļ�ѧ����ʽΪCH3COCH3+CH��CH$\stackrel{KOH}{��}$
 ��
����4��D��E�о�����̼̼˫���������D��˵������ȷ����ab��
a������ʽΪC5H12O
b���ɱ�������ȩ��
c�������ź���̼̼˫�����ǻ�
d���ܹ������ӳɡ���ȥ��ȡ�����ۺϵȷ�Ӧ
��5��H����˳���칹���䷴ʽ�ṹ��ʽΪ
 ��H��ͬ���칹�������ڷ����廯����Ĺ���5�֣�
��H��ͬ���칹�������ڷ����廯����Ĺ���5�֣���6��G��H�Ļ�ѧ����ʽΪ
 +CH3CHO$��_{��}^{NaOH}$
+CH3CHO$��_{��}^{NaOH}$ +H2O��
+H2O����7��B��FeCl3��Һ��������ɫ��B�� G��һ�������·�Ӧ����X�Ļ�ѧ����ʽΪ
 ��
��
���� �ڴ��������£���ϩ�ͱ������ӳɷ�Ӧ���� ��
�� ��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��
��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ�� ��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ��
��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ�� ��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ����
��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ���� ������H�Ľṹ��ʽΪ��
������H�Ľṹ��ʽΪ�� �����������Ϣ֪��G�Ľṹ��ʽΪ��
�����������Ϣ֪��G�Ľṹ��ʽΪ�� ��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ��
��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ��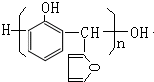 �������л���Ľṹ�����ʽ��
�������л���Ľṹ�����ʽ��
��� �⣺�ڴ��������£���ϩ�ͱ������ӳɷ�Ӧ���� ��
�� ��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��
��Ӧ����B��A��B��FeCl3��Һ��������ɫ������B�ķ���ʽ��֪��BΪ���ӣ�A����Ȳ�ܷ�����Ӧ����D��A�����к˴Ź����������շ�ֻ��1�飬����A�ķ���ʽ֪��A��CH3COCH3��CH3COCH3����Ȳ�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ�� ��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ��
��C�����������ӳɷ�Ӧ����D��D������ȥ��Ӧ����E�����F�Ľṹ��ʽ֪��E�Ľṹ��ʽΪ��CH2=CHC��CH3��=CH2��D�Ľṹ��ʽΪ�� ��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ����
��G����ȩ��Ӧ����H��H��������Һ��ӦȻ���ữ���� ������H�Ľṹ��ʽΪ��
������H�Ľṹ��ʽΪ�� �����������Ϣ֪��G�Ľṹ��ʽΪ��
�����������Ϣ֪��G�Ľṹ��ʽΪ�� ��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ��
��G�ͱ��ӷ�Ӧ����X����X�Ľṹ��ʽΪ�� ��
��
��1����������ķ�����֪���ٷ�Ӧ������Ϊ�ӳɷ�Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ��
��2����������ķ�����֪��A��CH3COCH3��
�ʴ�Ϊ��CH3COCH3��
��3��A��CΪCH3COCH3����Ȳ�����ӳɷ�Ӧ����C����Ӧ�Ļ�ѧ����ʽCH3COCH3+CH��CH$\stackrel{KOH}{��}$ ��
��
�ʴ�Ϊ��CH3COCH3+CH��CH$\stackrel{KOH}{��}$ ��
��
��4��D�Ľṹ��ʽΪ�� ��
��
a��D����ʽΪC5H10O����a����
b��D���ǻ�̼��û���⣬���ɱ�������ȩ�࣬��b����
c��D�й����ź���̼̼˫�����ǻ�����c��ȷ��
d��D����̼̼˫�����ǻ����ܹ������ӳɡ���ȥ��ȡ�����ۺϵȷ�Ӧ����d��ȷ��
��ѡ��ab��
��5��H�Ľṹ��ʽΪ�� ���䷴ʽ�ṹ��ʽΪ
���䷴ʽ�ṹ��ʽΪ  ��H��ͬ���칹�������ڷ����廯����ĽṹΪ����������-OH��-CHO�����ڼ�����֣�������-OOCH����-COOH������5�֣�
��H��ͬ���칹�������ڷ����廯����ĽṹΪ����������-OH��-CHO�����ڼ�����֣�������-OOCH����-COOH������5�֣�
�ʴ�Ϊ�� ��5��
��5��
��6��G�Ľṹ��ʽΪ�� ��G��H�Ļ�ѧ����ʽΪ
��G��H�Ļ�ѧ����ʽΪ +CH3CHO$��_{��}^{NaOH}$
+CH3CHO$��_{��}^{NaOH}$ +H2O��
+H2O��
�ʴ�Ϊ�� +CH3CHO$��_{��}^{NaOH}$
+CH3CHO$��_{��}^{NaOH}$ +H2O��
+H2O��
��7��BΪ���ӣ�B�� G��һ�������·�Ӧ����X�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϣ�ע���������Ϣ�������ơ��������ϵķ������з�����ע������ŵı仯���ѵ���ͬ���칹��������жϣ��ѶȽϴ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��CH3��3CCH2OH | B�� |  | C�� |  | D�� | ��CH3��2CBrCH2OH |
| A�� | ��ѧ�仯һ��������µĺ��� | |
| B�� | ������ˮ�����漰��ѧ�仯 | |
| C�� | �����ܸı仯ѧ��Ӧ�Ļ�ܺ��ʱ� | |
| D�� | ��ʵ����������м������ͭ��ϡ����Ϊԭ���Ʊ�ͭ������������;���� ;��a��Fe$\stackrel{H_{2}SO_{4}}{��}$H2$��_{��}^{Cu}$Cu����;��b��CuO$\stackrel{H_{2}SO_{4}}{��}$CuSO4$\stackrel{Fe}{��}$Cu ʵ�ʲ����У��Ƶõ�������ͭʱ��;��a��;��b����Fe������һ����� |
| A�� | ������ӵ����ģ�ͣ� | B�� | NH4Br�ĵ���ʽ�� | ||
| C�� | ��ԭ�ӵĽṹʾ��ͼ�� | D�� | ���ǻ�������Ľṹ��ʽ�� |
| A�� | ������Ϊ�л��� | B�� | ������Ϊ���� | ||
| C�� | ������������Ԫ����� | D�� | ������ȼ�ղ�����ܺ�CO2 |

 ��
�� �Т١��ڡ���3��-OH��������ǿ������˳���ǣ��ۣ��٣��ڣ�
�Т١��ڡ���3��-OH��������ǿ������˳���ǣ��ۣ��٣��ڣ�


 ��1��Ŀǰ��ҵ�ϳɰ���ԭ����N2��g��+3H2��g��?2NH3��g����H=-93.0kJ•mol-1��
��1��Ŀǰ��ҵ�ϳɰ���ԭ����N2��g��+3H2��g��?2NH3��g����H=-93.0kJ•mol-1��