��Ŀ����
2�� ��֪����A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����2CH3CHO+O2$��_{��}^{����}$2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
��֪����A��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ����2CH3CHO+O2$��_{��}^{����}$2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ���ش��������⣺
��1��д��A�Ľṹʽ
 ��
����2��B��D�����еĹ����ŵ����Ʒֱ����ǻ����Ȼ���
��3��д�����з�Ӧ�ķ�Ӧ���ͣ��ټӳɷ�Ӧ����������Ӧ����������Ӧ����ȡ����Ӧ����
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH�� ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O����CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
���� ����Ϣ��֪AΪCH2=CH2����Ϻϳ�·��ͼ��֪��A��ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH��B����������Ӧ����CΪCH3CHO��C����������Ӧ����DΪCH3COOH��B��D����������Ӧ����CH3COOCH2CH3���Դ������
��� �⣺����Ϣ��֪AΪCH2=CH2����Ϻϳ�·��ͼ��֪��A��ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH��B����������Ӧ����CΪCH3CHO��C����������Ӧ����DΪCH3COOH��B��D����������Ӧ����CH3COOCH2CH3��
��1��AΪCH2=CH2��A�ĽṹʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��B�й�����Ϊ�ǻ���D�й�����Ϊ�Ȼ����ʴ�Ϊ���ǻ����Ȼ���
��3����Ӧ�١��ڡ��ܵķ�Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��������Ӧ��������Ӧ����ȡ����Ӧ�����ʴ�Ϊ���ӳɷ�Ӧ��������Ӧ��������Ӧ����ȡ����Ӧ����
��4����Ӧ�١��ڡ��ֱܷ�ΪCH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬���չ����������ʵĹ�ϵ���ϳ������еķ�Ӧ���л���Ӧ����Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע�ⷴӦ�й����ŵı仯����Ŀ�ѶȲ���

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�| ѡ�� | �����ֵ����� | ʵ�鷽�� |
| A | �ƺͰ״� | �ٹ۲���ɫ������ζ |
| B | ʳ�κͰ��� | �ټ�ϡ����ڼ�ˮ�ܽ� |
| C | �ϳ���ά����Ȼ��ά | �ٵ�ȼ����ζ�ڹ۲�ɫ�� |
| D | ���Բ�����������Գ������� | �ٲ�pH�ڼ�ʯ��ʯ |
| A�� | A | B�� | B | C�� | C | D�� | D |
��Na2S+2HCl=2NaCl+H2S�� ��FeS+H2SO4��ϡ��=FeSO4+H2S��
��ZnS+2HCl=ZnCl2+H2S�� ��K2S+H2SO4��ϡ��=K2SO4+H2S��
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
| A�� | C8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��g����H=-48.40 kJ•mol-1 | |
| B�� | C8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��l����H=-5518 kJ•mol-1 | |
| C�� | C8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��l����H=+5518 kJ•mol-1 | |
| D�� | C8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��l����H=-48.40 kJ•mol-1 |

 ��
��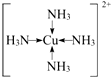 ����Ԫ�ط��ű�ʾ����
����Ԫ�ط��ű�ʾ����

 ��
��