��Ŀ����
Ϊ�ⶨijþ���Ͻ𣨼��費���������ʣ�����������������ij�о���ѧϰС�������һ������ʵ�鷽����
ʵ�鷽��һ����þ�Ͻ�
�ⶨʣ���������
��1���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��ʵ�������ӵ�����������ҺӦ�������������������������� ���ƫ�ߡ���ƫ�͡�����
��3�����Ƶ�þ���Ͻ�����Ϊm1������������������Һ��ַ�Ӧ���˳�ʣ����岢����ϴ�ӡ���ɣ��Ƶù�������Ϊm2g����������������Ϊ ��д����ѧ����ʽ����������δ��ϴ�ӣ������������������� ���ƫ�ߡ���ƫ�͡�����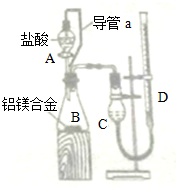
ʵ�鷽��������þ�Ͻ�
�ⶨ������������
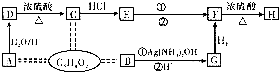
װ����ͼ��ʾ���ش��������⣺
��4��װ���е���a�������� ��
��5��ʵ������У�װ��������ɺ������²������裺
�ٽ�ҩƷ��ˮ�ֱ�װ���Ӧ������
�ڽ�A�����ᣨ����������B�У���B�в��ٲ�����������ȴ������
�ۼ��װ��������
�������ƶ�D��ʹC��DҺ����ƽ����¼D��Һ��λ��
�������������˳���� ������ţ����ظ���
��6��ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��B��C֮������һ��װ�м�ʯ�ҵĸ���װ�ã��������ǣ� �����Ҫ������Ҫ������
ʵ�鷽��һ����þ�Ͻ�
| ����������Һ |
��1���÷�Ӧ�Ļ�ѧ����ʽ��
��2��ʵ�������ӵ�����������ҺӦ��������������������������
��3�����Ƶ�þ���Ͻ�����Ϊm1������������������Һ��ַ�Ӧ���˳�ʣ����岢����ϴ�ӡ���ɣ��Ƶù�������Ϊm2g����������������Ϊ

ʵ�鷽��������þ�Ͻ�
| ���� |
װ����ͼ��ʾ���ش��������⣺
��4��װ���е���a��������
��5��ʵ������У�װ��������ɺ������²������裺
�ٽ�ҩƷ��ˮ�ֱ�װ���Ӧ������
�ڽ�A�����ᣨ����������B�У���B�в��ٲ�����������ȴ������
�ۼ��װ��������
�������ƶ�D��ʹC��DҺ����ƽ����¼D��Һ��λ��
�������������˳����
��6��ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��B��C֮������һ��װ�м�ʯ�ҵĸ���װ�ã��������ǣ�
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������1����������������Һ��Ӧ����ƫ�����ƺ�������
��2������������Һ���㣬������ȫ����Ӧ�ⶨ�����ƫ�ͣ�
��3����Ӧǰ����������仯Ϊ�ܽ�����������������ܽ����������õ�������������������δ��ϴ�ӣ�����մ�����ʣ�����õ�����������С���ⶨ������������ƫ�ͣ�
��4��a�ĵ�����ƽ��ѹǿ�����ã�����Һ�����£�
��5������ʵ�鲽�裬���ʵ����̺�Ŀ��д����ȷ�IJ���˳��
��6����Ӧ�����лӷ������Ȼ���������ˮ����Ӱ��ⶨ�����
��2������������Һ���㣬������ȫ����Ӧ�ⶨ�����ƫ�ͣ�
��3����Ӧǰ����������仯Ϊ�ܽ�����������������ܽ����������õ�������������������δ��ϴ�ӣ�����մ�����ʣ�����õ�����������С���ⶨ������������ƫ�ͣ�
��4��a�ĵ�����ƽ��ѹǿ�����ã�����Һ�����£�
��5������ʵ�鲽�裬���ʵ����̺�Ŀ��д����ȷ�IJ���˳��
��6����Ӧ�����лӷ������Ȼ���������ˮ����Ӱ��ⶨ�����
���
�⣺ʵ�鷽��һ����1����������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2��ʵ�������ӵ�����������ҺӦ�����������������ܽ���ȫ�������������������ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��3�����Ƶ�þ���Ͻ�����Ϊm1������������������Һ��ַ�Ӧ���˳�ʣ����岢����ϴ�ӡ���ɣ��Ƶù�������Ϊm2g����������������=
��100%��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ����������ƫС��������������ƫ�ͣ��ʴ�Ϊ��
��100%��ƫ�ͣ�
ʵ�鷽��������4��װ���е���a��������ƽ��ѹǿʹ��Һ©���е�Һ��˳�����£�
�ʴ�Ϊ��ƽ��ѹǿʹ��Һ©���е�Һ��˳�����£�
��5����Ӧ��װ�õ������ԡ��Ͻ��Ƿ���ȫ�ܽⶼ��Ӱ��ⶨ�����������Ҫ���װ�õ������ԣ������м����Լ���������������Ͻ���ȫ�ܽ⣬����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ��������ȷ�IJ���˳���ǣ��ۢ٢ڢܣ�
�ʴ�Ϊ���ۢ٢ڢܣ�
��6�������Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã��ʴ�Ϊ������Ҫ��
��2��ʵ�������ӵ�����������ҺӦ�����������������ܽ���ȫ�������������������ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��3�����Ƶ�þ���Ͻ�����Ϊm1������������������Һ��ַ�Ӧ���˳�ʣ����岢����ϴ�ӡ���ɣ��Ƶù�������Ϊm2g����������������=
| m1-m2 |
| m1 |
��100%��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ����������ƫС��������������ƫ�ͣ��ʴ�Ϊ��
| m1-m2 |
| m1 |
ʵ�鷽��������4��װ���е���a��������ƽ��ѹǿʹ��Һ©���е�Һ��˳�����£�
�ʴ�Ϊ��ƽ��ѹǿʹ��Һ©���е�Һ��˳�����£�
��5����Ӧ��װ�õ������ԡ��Ͻ��Ƿ���ȫ�ܽⶼ��Ӱ��ⶨ�����������Ҫ���װ�õ������ԣ������м����Լ���������������Ͻ���ȫ�ܽ⣬����������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ������ȴ�������ٶ��������ȷ�IJ���˳���ǣ��ۢ٢ڢܣ�
�ʴ�Ϊ���ۢ٢ڢܣ�
��6�������Ȼ��⼫������ˮ���ӷ����Ȼ��ⲻӰ����������IJⶨ��������Բ���Ҫ�ӳ���װ�ã��ʴ�Ϊ������Ҫ��
���������⿼����������ɷ�����ʵ��̽���ⶨ�����ͼ��㣬������������ע���������ʵķ�����ʵ���������������Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�������ʵ�ˮ��Һ�ܵ��磬�����ڷǵ���ʵ��ǣ�������
| A��HNO3 |
| B��C2H5OH |
| C��NH4NO3 |
| D��SO2 |
ȥ�������л��е����������ᣬӦѡ�õ���÷����ǣ�������
| A���ڻ�����м������ᣬ��������÷�Һ©������ |
| B���ڻ�����м���NaOH��Һ���������ͨ�����CO2���壬����ȫ��Ӧ���÷�Һ©������ |
| C������������������� |
| D���ڻ�����м������ѣ��������ȡ���ӣ�Ȼ�����÷�Һ©������ |
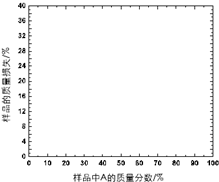 ������A��һ�����ȶ��Խϲ����ˮ���������Σ������·���������з�������A��������ϻ�Ͼ����Ƴ���Ʒ��������400�棬��¼��A����ͬ����Ʒ��������ʧ��%������������±�������������Ϣ���±������ݣ�ͨ����ͼ���ƶϻ�����A�Ļ�ѧʽ����������Ҫ������̣�
������A��һ�����ȶ��Խϲ����ˮ���������Σ������·���������з�������A��������ϻ�Ͼ����Ƴ���Ʒ��������400�棬��¼��A����ͬ����Ʒ��������ʧ��%������������±�������������Ϣ���±������ݣ�ͨ����ͼ���ƶϻ�����A�Ļ�ѧʽ����������Ҫ������̣�