��Ŀ����
7������ʯ��������õ�����[KAl��SO4��2•12H2O]���������Ʊ�Al��K2SO4��H2SO4�Ĺ��չ�����ͼ1��ʾ��
���������Ļ�ѧ����ʽΪ��4KAl��SO4��2•12H2O+3S=2K2SO4+2Al2O3+9SO2+48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У���ԭ����S��
��2����ˮ�������Һ�еõ�K2SO4����ķ����������ᾧ��
��3��A12O3��һ�������¿��Ƶ�AIN���侧��ṹ��ͼ2��ʾ���þ�����Al����λ����4
��4����Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO��OH��ת��ΪNi��OH��2���õ�ط�Ӧ�Ļ�ѧ����ʽ��Al+3NiO��OH��+H2O�TNaAlO2+3Ni��OH��2��
��5�����ղ�����SO2�����������ᣮ��֪25�桢101kPaʱ��
2SO2��g��+O2��g��?2SO3��g����H1=һ197kJ/mol��
2H2O ��g��=2H2O��1����H2=һ44kJ/mol��
2SO2��g��+O2��g��+2H2O��g��=2H2SO4��l����H3=һ545kJ/mol��
��SO3 ��g����H2O��l����Ӧ���Ȼ�ѧ����ʽ��SO3��g��+H2O��l���TH2SO4��l����H=-130KJ/mol��
����948t������M=474g/mol ������SO2��������Ϊ96%����������������Ϊ98%������432t��
���� ���������շ���4KAl��SO4��2•12H2O+3S=2K2SO4+2Al2O3+9SO2��+48H2O�������������������������������������������������������������������98.3%��Ũ�������գ�SO3+H2O=H2SO4���Ƶ�����������ù��������ˮ����Ϊ��߽����ʣ��ɲ�ȡ�������������Ͻ��裬��ˮ�������Һ�������ᾧ�õ�K2SO4���壬���Al2O3���Ƶ�Al���Դ˽����⣮
��1�����ݻ�ѧ����ʽ��Ԫ�ػ��ϼ۱仯�����жϣ�Ԫ�ػ��ϼ����ߵ�����ԭ������������Ӧ��
��2����ˮ�������Һ�еõ�K2SO4����ķ���������������ܽ�����¶ȱ仯���������������ܼ������ᾧ������
��3����λ��������ֱ�Ӻ�����ԭ�ӣ������ӣ�����ϵ����λԭ�ӵ���Ŀ��
��4����Al��NiO��OH��Ϊ�缫������ԭ��� ����ʧ���ӷ���������Ӧ��NiO��OH���õ����ӷ�����ԭ��Ӧ�����ԭ���غ�͵����غ�д����Ӧ��ѧ����ʽ��
��5�������Ȼ�ѧ����ʽ��˹���ɼ���õ���������Ԫ���غ����õ���
��� �⣺��1��4KAl��SO4��2•12H2O+3S�T2K2SO4+2Al2O3+9SO2+48H2O��Ӧ��������Ԫ�ػ��ϼ�����Ϊ+4�ۣ��������������Ԫ�ػ��ϼ۴�+6�۱仯Ϊ+4�ۣ���ԭ�������ʣ��ʴ�Ϊ��S��
��2����ˮ�������Һ�еõ�K2SO4����ķ���������������ܽ�����¶ȱ仯���������������ܼ������ᾧ�������壻�ʴ�Ϊ�������ᾧ��
��3�����ݾ��徧���ṹ�����������λ�������֪��ÿ����ԭ�Ӻ��ĸ���ԭ��������������ԭ�ӵ���λ��Ϊ4���ʴ�Ϊ��4��
��4����Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO��OH��ת��ΪNi��OH��2����������ʧ����������������Һ������ƫ�����ƣ���Ӧ��ѧ����ʽΪ��Al+3NiO��OH��+NaOH+H2O=NaAlO2+3Ni��OH��2 ���ʴ�Ϊ��Al+3NiO��OH��+NaOH+H2O=NaAlO2+3Ni��OH��2��
��5��2SO2��g��+O2��g��?2SO3��g����H1=-197kJ/mol��
H2O��g��?H2O��l����H2=-44kJ/mol��
2SO2��g��+O2��g��+2H2O��g���T2H2SO4��l����H3=-545kJ/mol��
���ݸ�˹���ɢ�-��-2����õ���2SO3��g��+2H2O��l��=2H2SO4��l����H=-260kJ/mol��
����Ӧ���Ȼ�ѧ����ʽΪ��SO3��g��+H2O��l��=H2SO4��l����H=-130kJ/mol��
948 t��������SO2���ʵ���Ϊ4500mol�����������������m=4500��0.96��98��0.98=432000kg=432t��
�ʴ�Ϊ��SO3��g��+H2O��l��=H2SO4��l����H=-130kJ/mol��432��
���� ���⿼�����ʵ��Ʊ���Ϊ�߿��������ͣ���Ŀ�Ѷ��еȣ��漰������ԭ��Ӧ��������жϡ�����ṹ�����⡢ԭ��ص�Ӧ�á��Ȼ�ѧ����ʽ��˹���ɵļ��㡢Ԫ���غ�ļ��㣬��������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�������������ʵ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����ͭ��Һ��H2S��Ӧ�����ӷ���ʽ��Cu2++S2-=CuS�� | |
| B�� | ��������Һ�����Ũ��ˮ��Ӧ�����ӷ���ʽ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+ | |
| C�� | H2��H2S��SO2��CO2�������嶼����Ũ������� | |
| D�� | SO2��SO3�������ͨ��Ba��NO3��2��Һ�ɵõ�BaSO3��BaSO4 |
 �����£�0.2mol•L-1NaHA ���Ũ�ȵ������ NaOH ��Һ��ϣ�������Һ�в�������ּ�Ũ����ͼ��ʾ������˵����ȷ���ǣ�������
�����£�0.2mol•L-1NaHA ���Ũ�ȵ������ NaOH ��Һ��ϣ�������Һ�в�������ּ�Ũ����ͼ��ʾ������˵����ȷ���ǣ�������| A�� | NaHA ��Һ������ | B�� | �����Һ�� c��OH-����c��H+����� | ||
| C�� | ͼ�� X��Y��Z �ֱ���� OH-��HA-��H+ | D�� | �����Һ�У�c��Na+���Tc��H2A��+c��HA-��+c��A2-�� |
| A�� | �л�ѧ�����ƻ��ı仯��һ���ǻ�ѧ�仯 | |
| B�� | ����Ԫ����ǽ���Ԫ��ԭ�Ӽ��γɵĻ�ѧ����һ�������Ӽ� | |
| C�� | ��Ȳ�����У��Ҽ���м���Ŀ֮��Ϊ1��1 | |
| D�� | ���ʯ��̼ԭ�Ӳ�ȡsp3�ӻ���12g���ʯ�к�2mol ̼̼���� |
| A�� | ����13.5% | B�� | ����12.5% | C�� | С��12.5% | D�� | ��ȷ�� |
| A�� | ����ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2�� | |
| B�� | ����NaHCO3��Һ��Ca��OH��2��Һ��Ӧ��OH��+HCO3��+Ca2+=CaCO3��+H2O | |
| C�� | �ƺ���ˮ��Ӧ��2Na+2H2O=2 Na++2OH��+H2�� | |
| D�� | ������CO2������������Һ�ķ�Ӧ OH��+CO32-=HCO3�� |
 ��
��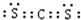 ���ṹʽ�ǣ�S=C=S��
���ṹʽ�ǣ�S=C=S��





 ��
�� ��
�� ��
�� ���ڡ��䡢����дһ�ּ��ɣ���
���ڡ��䡢����дһ�ּ��ɣ���