��Ŀ����
9���ϳɾ���ϩ����PF��֬�;�����άPET��·�����£�
��֪����
 ��RΪHԭ�ӻ�������
��RΪHԭ�ӻ���������RCOOR��+R��OH$?_{����}^{����}$ RCOO R��+R��OH��R��R�䡢R�����������
�ش��������⣺
��1��A�ĺ˴Ź���������3�����շ壬A�Ľṹ��ʽΪCH3CH2OH
��2��B�Ĺ������������Ȼ�
��3��X���ɵ�ʯ�ͱ���ʳ��ˮ��Ӧ���ɵ�����B��X�����ʵ���֮��1��1�����ӳɷ�Ӧ����C����
C��D�ķ�Ӧ�����ǼӾ۷�Ӧ
��4��Y�Ǻϳ�PF��֬��һ�ֵ��壬Y�Ľṹ��ʽΪ

��5��A��E�Ļ�ѧ����ʽΪ

��6��
 ��ͬ���칹��WҲ�ܱ����Ը��������Һ����ΪG��W�����п��ܽṹ��ʽΪ
��ͬ���칹��WҲ�ܱ����Ը��������Һ����ΪG��W�����п��ܽṹ��ʽΪ
��7��Z��A��Ϊͬϵ�Z����Է���������AС14��G��I�Ļ�ѧ����ʽΪ

��8����֪��A$��_{170��}^{ŨH_{2}SO_{4}}$$\stackrel{Br_{2}/CCl_{4}}{��}$$��_{��}^{NaOH��Һ}$Q��Q�ķе����A������Q������������Һ��Q�Ľṹ��ʽ��HOCH2CH2OH
��9��I��PET�Ļ�ѧ����ʽΪ
 ��
��
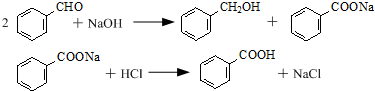
���� A�ĺ˴Ź���������3�����շ壬��A�ķ���ʽ��֪��AΪCH3CH2OH��A��������EΪCH3CHO������PF��֬�Ľṹ��֪��YΪ ��A���ظ����������BΪCH3COOH��X���ɵ�ʯ�ͱ���ʳ��ˮ��Ӧ���ɵ�����B��X�����ʵ���֮��1��1�����ӳɷ�Ӧ����C������XΪCH��CH��CΪCH3COOCH=CH2��C�����Ӿ۷�Ӧ��DΪ
��A���ظ����������BΪCH3COOH��X���ɵ�ʯ�ͱ���ʳ��ˮ��Ӧ���ɵ�����B��X�����ʵ���֮��1��1�����ӳɷ�Ӧ����C������XΪCH��CH��CΪCH3COOCH=CH2��C�����Ӿ۷�Ӧ��DΪ ��D�ٷ���ˮ�ⷴӦ���ɾ���ϩ����A��Ũ�廯�ⷴӦ����FΪCH3CH2Br���Զ��ұ�����������GΪ
��D�ٷ���ˮ�ⷴӦ���ɾ���ϩ����A��Ũ�廯�ⷴӦ����FΪCH3CH2Br���Զ��ұ�����������GΪ ��Z��A��Ϊͬϵ�Z����Է���������AС14����ZΪCH3OH��G��Z����������Ӧ����IΪ
��Z��A��Ϊͬϵ�Z����Է���������AС14����ZΪCH3OH��G��Z����������Ӧ����IΪ ��I��P�ڴ������ȵ������·���������Ϣ���еķ�Ӧ���ɾ�����άPET������A$��_{170��}^{ŨH_{2}SO_{4}}$$\stackrel{Br_{2}/CCl_{4}}{��}$$��_{��}^{NaOH��Һ}$Q��Q�ķе����A������Q������������Һ������QΪHOCH2CH2OH��PETΪ
��I��P�ڴ������ȵ������·���������Ϣ���еķ�Ӧ���ɾ�����άPET������A$��_{170��}^{ŨH_{2}SO_{4}}$$\stackrel{Br_{2}/CCl_{4}}{��}$$��_{��}^{NaOH��Һ}$Q��Q�ķе����A������Q������������Һ������QΪHOCH2CH2OH��PETΪ ���ݴ˴��⣻
���ݴ˴��⣻
��� �⣺A�ĺ˴Ź���������3�����շ壬��A�ķ���ʽ��֪��AΪCH3CH2OH��A��������EΪCH3CHO������PF��֬�Ľṹ��֪��YΪ ��A���ظ����������BΪCH3COOH��X���ɵ�ʯ�ͱ���ʳ��ˮ��Ӧ���ɵ�����B��X�����ʵ���֮��1��1�����ӳɷ�Ӧ����C������XΪCH��CH��CΪCH3COOCH=CH2��C�����Ӿ۷�Ӧ��DΪ
��A���ظ����������BΪCH3COOH��X���ɵ�ʯ�ͱ���ʳ��ˮ��Ӧ���ɵ�����B��X�����ʵ���֮��1��1�����ӳɷ�Ӧ����C������XΪCH��CH��CΪCH3COOCH=CH2��C�����Ӿ۷�Ӧ��DΪ ��D�ٷ���ˮ�ⷴӦ���ɾ���ϩ����A��Ũ�廯�ⷴӦ����FΪCH3CH2Br���Զ��ұ�����������GΪ
��D�ٷ���ˮ�ⷴӦ���ɾ���ϩ����A��Ũ�廯�ⷴӦ����FΪCH3CH2Br���Զ��ұ�����������GΪ ��Z��A��Ϊͬϵ�Z����Է���������AС14����ZΪCH3OH��G��Z����������Ӧ����IΪ
��Z��A��Ϊͬϵ�Z����Է���������AС14����ZΪCH3OH��G��Z����������Ӧ����IΪ ��I��P�ڴ������ȵ������·���������Ϣ���еķ�Ӧ���ɾ�����άPET������A$��_{170��}^{ŨH_{2}SO_{4}}$$\stackrel{Br_{2}/CCl_{4}}{��}$$��_{��}^{NaOH��Һ}$Q��Q�ķе����A������Q������������Һ������QΪHOCH2CH2OH��PETΪ
��I��P�ڴ������ȵ������·���������Ϣ���еķ�Ӧ���ɾ�����άPET������A$��_{170��}^{ŨH_{2}SO_{4}}$$\stackrel{Br_{2}/CCl_{4}}{��}$$��_{��}^{NaOH��Һ}$Q��Q�ķе����A������Q������������Һ������QΪHOCH2CH2OH��PETΪ ��
��
��1����������ķ�����֪��A�Ľṹ��ʽΪ CH3CH2OH��
�ʴ�Ϊ��CH3CH2OH��
��2��BBΪCH3COOH���Ĺ������������Ȼ���
�ʴ�Ϊ���Ȼ���
��3����������ķ�����֪��C��D�ķ�Ӧ������ �Ӿ۷�Ӧ��
�ʴ�Ϊ���Ӿ۷�Ӧ��
��4��Y��������ķ�����֪��Y�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5��A��������������E��A��E�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6�� ��ͬ���칹��WҲ�ܱ����Ը��������Һ����ΪG��˵���뱽��������̼������ԭ�ӣ������������W�����п��ܽṹ��ʽΪ
��ͬ���칹��WҲ�ܱ����Ը��������Һ����ΪG��˵���뱽��������̼������ԭ�ӣ������������W�����п��ܽṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��7��GΪ ��G��I�Ļ�ѧ����ʽΪ
��G��I�Ļ�ѧ����ʽΪ  ��
��
�ʴ�Ϊ�� ��
��
��8����������ķ�����֪��Q�Ľṹ��ʽ��HOCH2CH2OH��
�ʴ�Ϊ��HOCH2CH2OH��
��9��I��PET�Ļ�ѧ����ʽΪ  ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬����PMMA�ۺ���ṹ��ʽ��Ϸ�Ӧ������������˼ά���������ƶϣ���ȷ�ƶ����ʽṹ��ʽ�ǽⱾ��ؼ���ע��֪ʶ��������ã���Ŀ�ѶȲ���

| A�� | �����Һ������ | |
| B�� | a��b | |
| C�� | �����Һ�У�c��A-��=c��Na+�� | |
| D�� | ���Һ��ˮ�����c��OH-�����ڸ��¶��´�ˮ�����c��OH-�� |
������ʵIJ����������ʼ��±���
| ���� | ����ܶ� | �۵㣨�棩 | �е㣨�棩 | �ܽ�� | |
| ˮ | ���� | ||||
| ����ȩ | 1.04 | -26 | 179.6 | �� | ���� |
| ������ | 1.27 | 122.1 | 249 | 25���ܣ�95����� | ���� |
| ���״� | 1.04 | -15.3 | 205.7 | �� | ���� |
| ���� | 0.71 | -116.3 | 34.6 | ���� | -- |

��1����ȡʱ���״��ڷ�Һ©�����ϲ㣨��ϡ����¡����㣬��Һ©����ҡ�����������������������Σ���ԭ�����ŷſ��ܲ����������Խ����ѹ��
��2����NaHSO3��Һ��10%Na2CO3��Һ��H2Oϴ�����Ѳ㣮
����10%Na2CO3��Һϴ��Ŀ���dz�ȥ�������ܽ�����������ᣮ
�ڲ���������������
��3�����˲�������ʱ�Ⱥ���еIJ������ȶϿ�����ƿ������õ����ӣ���رճ����ã�
��4���ᴿ�ֲ�Ʒ�ҵ�ʵ�鷽��Ϊ�ؽᾧ��
| A�� | �� | B�� | ���ڵ��������� | C�� | ʳ��ˮ | D�� | ���� |
| A�� | ���������۵�ߣ�Ӳ�ȴ�����ˮ�ࡢ�մ��� | |
| B�� | ���������������쾧��ܡ����ɵ�· | |
| C�� | ����������������ʯӢ�ӱ���ѹ����Ϻ��ά | |
| D�� | ������������������������������ |
| A�� | �������������������β���Ĵ�����������������߿������� | |
| B�� | ���ٻ�ʯȼ�ϵ�ʹ�ã������ڽ��Ϳ�����PM2.5�ĺ��� | |
| C�� | ʳƷ��װ���г�����С������ʯ�ң�Ŀ���Ƿ�ֹʳƷ�������� | |
| D�� | ҽ�þƾ����õ�����ֲ��;������Ƴɣ�Ũ��ͨ����75% |
| A�� | ����������Ӧˮ��������ԣ�W��Z��X | |
| B�� | ������Q��ǿ������ | |
| C�� | Z��X�������γ����ֳ��������������� | |
| D�� | ����Ԫ�صij���������X�ĵ��ʵķе���� |
 ʵ������ȡ�����������װ����ͼ��ʾ�������������������գ�
ʵ������ȡ�����������װ����ͼ��ʾ�������������������գ�