��Ŀ����
20��ˮ�����������������Ҫ��Դ��Ҳ������������Ҫ����ɲ��֣���1����ʱӲˮ��Ӳ������̼�������̼����þ����ģ�Ӳˮ���Ⱥ�������������ӷ���ʽΪCa2++2HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O�ȣ�д������һ�ֳ�����ļ��ɣ���
��2��ij�о���С��ģ��ԭˮ����������ˮ�Ĺ�������ʾ��ͼ��ͼ1��

��ͨ�������̼��Ŀ���dz�ȥ������Ca2+ �͵���pH��
�����嵥��A���ҹ����õ�һ����������д��A��ˮ��Ӧ�����ӷ���ʽ��Cl2+H2O=H++Cl-+HClO��Ŀǰ�кܶ�A�����Ʒ��д���������ֵĻ�ѧʽCa��ClO��2��ClO2��K2FeO4�ȣ�
��3������ˮ�������Ʊ�����ˮ��ȥ����ˮ���Ĺ�������ʾ��ͼ��ͼ2��
�ٻ���̿������������ˮ�е��л����ȥ����ζ����
��A��B�з��õ����ʵ����ƣ�A�������ӽ�����֬��B�������ӻ���֬�� A��B�з��õ����ʲ����Ի�������˵��ԭ��Ӳˮ�е�Ca2+��Mg2+���ӻ��������ӽ�����֬�е�OH-����Mg��OH��2��Ca��OH��2�������������Ӱ����֬����Ч����
��4��ͨ��ʩ��һ��ѹ��ʹˮ����ͨ����Ĥ��������ӻ����ӽ������Ӷ���ô���ˮ�ķ�����Ϊ��������
��5����������ˮ�Ĵ���ʱ��������еķ����Dzⶨˮ�ĵ絼�ʣ�������ʣ���
���� ��1��̼����ơ�̼����þ�ֽ�����̼���Ρ�������̼��ˮ��
��2��ԭˮ���������м����������ƻ����þ��������������þ���������Ժ�̼��������ӷ�Ӧ����̼������Ӻ�ˮ����Ӧ�����ӷ���ʽΪ��HCO3-+OH-�TCO32-+H2O��Mg2++2OH-�TMg��OH��2������һ��������ͨ�������̼���壬�ɳ�ȥ������ʯ�ң�������ҺpH����������������ͨ������ɱ��������
�ٶ�����̼����ʯ��ˮ��Ӧ�����ɳ����������ڵ�����ҺpH��
��������ˮ��Ӧ����HCl��HClO��������Ca��ClO��2��ClO2��K2FeO4�ȴ���������
��3���ٻ���̿���������ԣ�
����ͨ�������ӽ�����֬��������Mg��OH��2�ȳ�����Ӱ����֬����Ч����
��4��ͨ��ʩ��һ��ѹ��ʹˮ����ͨ����Ĥ��������ӻ����ӽ������Ӷ���ô���ˮ�ķ�����Ϊ��������
��5����Һ�ĵ�������ȡ������Һ�е�����Ũ�ȴ�С����������ˮ�Ĵ���ʱ����ķ����Dzⶨˮ�ĵ����ʣ�
��� �⣺��1��Ӳˮ���Ⱥ�������������ӷ���ʽΪ��Ca2++2HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O��Mg2++2HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$MgCO3��+CO2��+H2O�ȣ�
�ʴ�Ϊ��Ca2++2HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O�ȣ�
��2��ԭˮ���������м����������ƻ����þ��������������þ���������Ժ�̼��������ӷ�Ӧ����̼������Ӻ�ˮ����Ӧ�����ӷ���ʽΪ��HCO3-+OH-�TCO32-+H2O��Mg2++2OH-�TMg��OH��2������һ��������ͨ�������̼���壬�ɳ�ȥ������ʯ�ң�������ҺpH����������������ͨ������ɱ��������
�ٶ�����̼����ʯ��ˮ��Ӧ�����ɳ����������ڵ�����ҺpH��
�ʴ�Ϊ����ȥ������Ca2+������pH��
��������ˮ��Ӧ����HCl��HClO����Ӧ���ӷ���ʽΪ��Cl2+H2O=H++Cl-+HClO��������Ca��ClO��2��ClO2��K2FeO4�ȴ���������
�ʴ�Ϊ��Cl2+H2O=H++Cl-+HClO��Ca��ClO��2��ClO2��K2FeO4�ȣ�
��3���ٻ���̿���������ԣ�����������ˮ�е��л����ȥ����ζ����
�ʴ�Ϊ������ˮ�е��л����ȥ����ζ����
��AΪ�����ӽ�����֬��BΪ�����ӽ�����֬��������Ӳˮ�е�Ca2+��Mg2+���ӻ��������ӽ�����֬�е�OH-����Mg��OH��2��Ca��OH��2�������������Ӱ����֬����Ч�������ܽ�����
�ʴ�Ϊ�����ܣ�Ӳˮ�е�Ca2+��Mg2+���ӻ��������ӽ�����֬�е�OH-����Mg��OH��2��Ca��OH��2�������������Ӱ����֬����Ч����
��4��ͨ��ʩ��һ��ѹ��ʹˮ����ͨ����Ĥ��������ӻ����ӽ������Ӷ���ô���ˮ�ķ�����Ϊ��������
�ʴ�Ϊ����������
��5����Ϊˮ�ĵ���̶ȼ�С�����Դ�ˮ�Ǽ���������ģ����Ҫ��������ˮ�Ĵ���ʱ��������еķ����Dzⶨˮ�ĵ絼�ʻ�����ʣ�
�ʴ�Ϊ���絼�ʣ�������ʣ���
���� ���⿼�����ڻ�ѧ�뼼�����漰ˮ�ľ�����Ӳˮ�����ȣ�ע�����ӽ�����֬�Ʊ�����ˮ��ע��Ի���֪ʶ���������գ�

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�| A�� | �������Խ��������Խ�� | |
| B�� | ԭ�Ӻ�����ӵ��˶�û�й��� | |
| C�� | �ڶ����ԭ���У�������ӷֲ��Ų� | |
| D�� | ����һ�㾡�����Ų��������ߵĵ��Ӳ��� |
����һ��

���̶���

����˵����ȷ���ǣ�������
| A�� | ��Ӧ�١��۵�ԭ�������ʾ�Ϊ100% | |
| B�� | �����������շ���HCOOH����ϴ��Һ��Һ�� | |
| C�� | ��ԭ�ϳ�����ýǶȿ������̶�������һ���� | |
| D�� | ���������ܷ�Ӧ��ΪCO+H2O��HCOOH |
| A�� | ԭ�Ӿ����й��ۼ�Խǿ���۵�Խ�� | |
| B�� | ���Ӿ����з��Ӽ�������Խ����Խ�ȶ� | |
| C�� | ���ۻ�ʱˮ�����й��ۼ��������� | |
| D�� | CaCl2�����к������ֻ�ѧ�� |

| A�� | ���Ӿ����ߴ�пƬ�����Ҳ�̼�����ٴ����̼������ͭƬ | |
| B�� | ͭƬ�Ϸ���������Ӧ | |
| C�� | �Ҳ�̼���Ϸ����ķ�Ӧ��2H++2e��H2�� | |
| D�� | ͭ�缫�������� |
| A�� | ��Al2��S04��3��Һ�м��������ˮ��A3++4NH3•H2O�TAlO-+4NH4++2H2O | |
| B�� | ����˫��ˮ��������KI��Һ��2H202+2I-�T2H++4H20+I2 | |
| C�� | ��������������ϡ���3Fe2++4H++N03-�T3Fe3++NO+2H2O | |
| D�� | ����п�̵��������Ӧ��2MnO2+2H2O+2eһ�T2MnOOH+2OH- |

| A�� | �����ж��Ե缫C1Ϊ���� | |
| B�� | �ù����ŵ�֮һ��FeCl3��Һ��ѭ������ | |
| C�� | ��Ӧ���е����ӷ���ʽ��2Fe3++S2-=2Fe2++S�� | |
| D�� | �����ܷ�Ӧ�Ļ�ѧ����ʽ��2FeCl2+2HCl$\frac{\underline{\;ͨ��\;}}{\;}$2FeCl3+H2�� |
 �������������ϳ���֬���ϳ��ȣ��Ա�ϩΪԭ���Ʊ�����J�ĺϳ�·����ͼ��
�������������ϳ���֬���ϳ��ȣ��Ա�ϩΪԭ���Ʊ�����J�ĺϳ�·����ͼ��
 +2NaOH$��_{��}^{H_{2}O}$
+2NaOH$��_{��}^{H_{2}O}$ +2NaCl��
+2NaCl��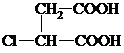 ��G�����к��еĹ��������Ȼ���̼̼˫��������������ƣ���
��G�����к��еĹ��������Ȼ���̼̼˫��������������ƣ���