��Ŀ����
1����0.1molij�������������״����4.48L��������ܱ���һ�����У�����������ȫȼ�գ��õ�CO2��CO��H2O����̬�������������ͨ��Ũ����ʱ��Ũ��������������3.6g��ͨ������ʯ��ˮʱ���ɵõ�����10g ��������������ʣ�����������ȵ���������ַ�Ӧ����ͨ������ʯ��ˮ�У��ֵõ�20g�������ʣ����������1�����л���ķ���ʽ��
��2�����л�����봼����������Ӧ���ҿ�ʹ��ˮ��ɫ��д���л���Ľṹʽ��
���� ��1��Ũ���������������3.6g��������ˮ��������3.6g��ͨ����������ʯ��ˮ���ɵþ������ij���10g���ݴ˿��Լ������ɶ�����̼��������ʣ������һ����̼���Ժ����ȵ���������ַ�Ӧ�����ɽ������Ͷ�����̼����ͨ�����ʯ��ˮʱ���ɸ������ɳ���������������ɶ�����̼���������������һ����̼������������Ԫ���غ���Լ����л����ʵķ���ʽ��
��2�����ݸ��л�����е����ʼ�����ʽȷ����ṹ��ʽ��
��� �⣺��1����״����4.48L���������ʵ���Ϊ��$\frac{4.48L}{22.4L/mol}$=0.2mol��
0.1mol�����������0.2mol������Ӧ������ˮ��������3.6g������ˮ�����ʵ����ǣ�$\frac{3.6g}{18g/mol}$=0.2mol�������л�����H�����ʵ���Ϊ0.4mol��ͨ����������ʯ��ˮ���ɵø����ij���10g������CO2��CaCO3��֪������̼�����ʵ����ǣ�$\frac{10g}{100g/mol}$=0.1mol��һ����̼�����ȵ���������ַ�Ӧ����ͨ�����ʯ��ˮʱ���ɵþ������ij���20g������CO��CO2��CaCO3��֪����һ����̼�����ʵ����ǣ�$\frac{20g}{100g/mol}$=0.2mol�����л����к�C�����ʵ����ǣ�0.1mol+0.2mol=0.3mol��
������ԭ���غ㣬������ԭ�ӵ����ʵ���Ϊ��0.2mol+0.1mol��2+0.2mol-0.2mol��2=0.2mol��
�����л����к���C��H��O��ԭ�Ӹ����ֱ�Ϊ��N��C��=$\frac{0.3mol}{0.1mol}$=3��N��H��=$\frac{0.4mol}{0.1mol}$=4��n��O��=$\frac{0.2mol}{0.1mol}$=2������л���ķ���ʽΪ��C3H4O2��
�𣺸��л���Ļ�ѧʽΪC3H4O2��
��2���л�����봼����������Ӧ�������Ȼ����ҿ�ʹ��ˮ��ɫ������̼̼˫���������л���Ľṹ��ʽΪ��CH2=CHCOOH��
�𣺸��л���Ľṹ��ʽΪCH2=CHCOOH��
���� ���⿼���л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ�ע��������غ�ĽǶȼ��㣬��ȷ�����л���ṹ������Ϊ���ؼ�������������ѧ���Ļ�ѧ����������

| A�� | Na+��H+��Cl-��SO42- | B�� | Na+��H+��Cl-��HCO3- | ||
| C�� | Al3+��K+��OH-��NO3- | D�� | NH4+��OH-��NO3-��Na+ |
| A�� | ����ɫ��NO2��ѹ����ɫ�ȱ�����dz | |
| B�� | �����ڳ�ʪ�Ŀ������������� | |
| C�� | �¶ȹ��߶Ժϳɰ����� | |
| D�� | �����£���1mLpH=3�Ĵ�����Һ��ˮϡ����l00mL�������pH��5 |
| A�� | ij����Ԫ����̬��̬ԭ�ӵ������ܵ���ֵ�ֱ�Ϊ738��1451��7733��10540��13630��17995��21703����������������Ӧʱ���ɵ���������X3+ | |
| B�� | �ֹ�$��_{����}^{Cl_{2}}$SiCl4$��_{����}^{H_{2}}$Si����һ��ʵ�� | |
| C�� | 33gCH��C-CH=CH-CH3�������Ħм�����12gʯī��������̼̼������Ϊ1.5mol | |
| D�� | ���հ�ɫ��ĩ������ɻ�ɫ��֤��ԭ��ĩ����Na+�����ܺ���K+ |
| A�� | CO��g�� ��Na2O2��s����Ӧ�ų�509kJ����ʱ������ת����Ϊ6.02��1023 | |
| B�� | 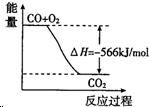 ͼ�ɱ�ʾ��CO����CO2�ķ�Ӧ���̺�������ϵ | |
| C�� | 2Na2O2��s��+2CO2��s���T2Na2CO3��s��+O2��g����H��-452kJ/mol | |
| D�� | CO��ȼ����Ϊ283kJ |
| A�� | NH4+ Fe3+ SO42- NO3- | B�� | K+ Na+ CO32- NO3- | ||
| C�� | K+ NH4+ OH- SO42- | D�� | Na+ K+ AlO2- Cl- |

| A�� | H+H��H-H | B�� | H-C1��H+C1 | ||
| C�� | Mg+2HCl�TMgCl2+H2�� | D�� | H2SO4+2NaOH�TNa2SO4+2H2O |
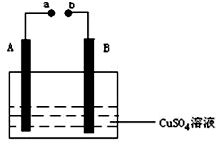 ��ͼ��ʾװ��Ϊ��ֱ����������µ��CuSO4��Һͼ������A��BΪʯī�缫��a��bΪ��Դ������������ͨ��Դ��ͨ��һ��ʱ���B�缫ȡ��ϴ�ɾ�����������������������3.2g����
��ͼ��ʾװ��Ϊ��ֱ����������µ��CuSO4��Һͼ������A��BΪʯī�缫��a��bΪ��Դ������������ͨ��Դ��ͨ��һ��ʱ���B�缫ȡ��ϴ�ɾ�����������������������3.2g����