��Ŀ����
ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��M��W������X��Z��M��W����Ԫ�ص�ԭ������֮��Ϊ32�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ�Ԫ��Yԭ�ӵ�����������������Ӳ�����2����Z��M�������ڣ�M��Wλ��ͬ���壮
��1��Ԫ��W�����ڱ��е�λ���� ��W�������ӽṹʾ��ͼ�� ��
��2��Ԫ��Z�ĵ��ʵĵ���ʽΪ ������£��Թ����ռ���Z���⻯�������ˮ�У����ʲ���ɢ����һ��ʱ����Թ�����Һ�����ʵ���Ũ��Ϊ ��
��3��Ԫ��Y������ͬ���������� �� Y����������еĻ�ѧ������Ϊ ��Y���⻯���к�������ߵ������dz�����ȼ�ϣ�1g��ȼ��ȼ�ղ���CO2��g����H2O��l��ʱ����55.6kJ����д����ʾ��ȼ��ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ
��4����X��Z��M��W����Ԫ���е�������ɵ�һ��ǿ�ᣬ�����ϡ��Һ����ͭ��Ӧ�����ӷ���ʽΪ
��5����X��Z��M��W��Fe����Ԫ����ɵ�Ħ������Ϊ392g/mol�Ļ�����ף�1mol���к���6mol�ᾧˮ���Ի������������ʵ�飺
a��ȡ����Һ���������ŨNaOH��Һ�����ȣ�������ɫ��������ɫ�̼�����ζ���壮��һ��ʱ���ɫ������Ϊ����ɫ�����ձ�Ϊ���ɫ��
b����ȡ����Һ���������BaCl2��Һ������ɫ������������������ܽ�
�ټĻ�ѧʽΪ ��
����֪100mL 1mol/L �ļ���Һ����20mL 1mol/L������KMnO4��Һǡ�÷�Ӧ��д����Ӧ�����ӷ���ʽ ��
��1��Ԫ��W�����ڱ��е�λ����
��2��Ԫ��Z�ĵ��ʵĵ���ʽΪ
��3��Ԫ��Y������ͬ����������
��4����X��Z��M��W����Ԫ���е�������ɵ�һ��ǿ�ᣬ�����ϡ��Һ����ͭ��Ӧ�����ӷ���ʽΪ
��5����X��Z��M��W��Fe����Ԫ����ɵ�Ħ������Ϊ392g/mol�Ļ�����ף�1mol���к���6mol�ᾧˮ���Ի������������ʵ�飺
a��ȡ����Һ���������ŨNaOH��Һ�����ȣ�������ɫ��������ɫ�̼�����ζ���壮��һ��ʱ���ɫ������Ϊ����ɫ�����ձ�Ϊ���ɫ��
b����ȡ����Һ���������BaCl2��Һ������ɫ������������������ܽ�
�ټĻ�ѧʽΪ
����֪100mL 1mol/L �ļ���Һ����20mL 1mol/L������KMnO4��Һǡ�÷�Ӧ��д����Ӧ�����ӷ���ʽ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺
������ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��M��W�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ���XΪHԪ�أ�Ԫ��Yԭ�ӵ�����������������Ӳ�����2����C��S���ϣ����ԭ��������֪��Y������ΪSԪ�أ���YΪCԪ�أ�Z��M�������ڣ�M��Wλ��ͬ���壬��Z��ԭ������Ϊa����֪Mԭ������Ϊa+1��Wԭ������Ϊa+9��X��Z��M��W����Ԫ�ص�ԭ������֮��Ϊ32����1+a+a+1+a+9=32�����a=7����ZΪNԪ�ء�MΪOԪ�ء�WΪSԪ�أ��ݴ˽��
���
�⣺ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��M��W�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ���XΪHԪ�أ�Ԫ��Yԭ�ӵ�����������������Ӳ�����2����C��S���ϣ����ԭ��������֪��Y������ΪSԪ�أ���YΪCԪ�أ�Z��M�������ڣ�M��Wλ��ͬ���壬��Z��ԭ������Ϊa����֪Mԭ������Ϊa+1��Wԭ������Ϊa+9��X��Z��M��W����Ԫ�ص�ԭ������֮��Ϊ32����1+a+a+1+a+9=32�����a=7����ZΪNԪ�ء�MΪOԪ�ء�WΪSԪ�أ�
��1��Ԫ��WΪS�������ڱ��е�λ���ǣ��������ڢ�A�壬S2-���ӽṹʾ��ͼ�� ���ʴ�Ϊ���������ڢ�A�壻
���ʴ�Ϊ���������ڢ�A�壻 ��
��
��2��ZΪNԪ�أ����ʵĵ���ʽΪ ������£��Թ����ռ���Z���⻯�������ˮ�У����ʲ���ɢ����һ��ʱ��������������Һ������������Ϊ1L�����Թ�����Һ�����ʵ���Ũ��Ϊ
������£��Թ����ռ���Z���⻯�������ˮ�У����ʲ���ɢ����һ��ʱ��������������Һ������������Ϊ1L�����Թ�����Һ�����ʵ���Ũ��Ϊ
=0.045mol/L��
�ʴ�Ϊ�� ��0.045mol/L��
��0.045mol/L��
��3��YΪCԪ�أ�������ͬ����������ʯī�����ʯ��C60�ȣ�C���������Ϊ������̼�������еĻ�ѧ�����ͼ��Թ��ۼ����⻯���к�������ߵ������dz�����ȼ�ϣ�������ΪCH4��1g��ȼ��ȼ�ղ���CO2��g����H2O��l��ʱ����55.6kJ����1mol������ȫȼ�շų�������Ϊ55.6kJ��
=889.6kJ���ʼ���ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol���ʴ�Ϊ��ʯī�����ʯ��C60�� ���Թ��ۼ��ۼ���CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��4����H��N��O��S����Ԫ���е�������ɵ�һ��ǿ�ᣬ�����ϡ��Һ����ͭ��Ӧ��Ӧ��ϡ������Cu��Ӧ��������ͭ��NO��ˮ����Ӧ���ӷ���ʽΪ��3Cu+8H++2NO3-=2Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=2Cu2++2NO��+4H2O��
��5��ȡ����Һ���������ŨNaOH��Һ�����ȣ�������ɫ��������ɫ�̼�����ζ���壮��һ��ʱ���ɫ������Ϊ����ɫ�����ձ�Ϊ���ɫ����˵���������������Ӻ�笠����ӣ���ȡ����Һ���������BaCl2��Һ������ɫ������������������ܽ⣬��˵����������������ӣ�1mol���к���6mol�ᾧˮ����ÿ�����к���6���ᾧˮ����Ħ������Ϊ392g/mol����Ļ�ѧʽΪ��NH4��2Fe��SO4��2?6H2O����NH4��2Fe��SO4��2������KMnO4��Һ��Ӧ�����ӷ���ʽΪ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O���ʴ�Ϊ���٣�NH4��2Fe��SO4��2?6H2O����5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
��1��Ԫ��WΪS�������ڱ��е�λ���ǣ��������ڢ�A�壬S2-���ӽṹʾ��ͼ��
 ���ʴ�Ϊ���������ڢ�A�壻
���ʴ�Ϊ���������ڢ�A�壻 ��
����2��ZΪNԪ�أ����ʵĵ���ʽΪ
 ������£��Թ����ռ���Z���⻯�������ˮ�У����ʲ���ɢ����һ��ʱ��������������Һ������������Ϊ1L�����Թ�����Һ�����ʵ���Ũ��Ϊ
������£��Թ����ռ���Z���⻯�������ˮ�У����ʲ���ɢ����һ��ʱ��������������Һ������������Ϊ1L�����Թ�����Һ�����ʵ���Ũ��Ϊ
| ||
| 1L |
�ʴ�Ϊ��
 ��0.045mol/L��
��0.045mol/L����3��YΪCԪ�أ�������ͬ����������ʯī�����ʯ��C60�ȣ�C���������Ϊ������̼�������еĻ�ѧ�����ͼ��Թ��ۼ����⻯���к�������ߵ������dz�����ȼ�ϣ�������ΪCH4��1g��ȼ��ȼ�ղ���CO2��g����H2O��l��ʱ����55.6kJ����1mol������ȫȼ�շų�������Ϊ55.6kJ��
| 1mol��16g/mol |
| 1g |
��4����H��N��O��S����Ԫ���е�������ɵ�һ��ǿ�ᣬ�����ϡ��Һ����ͭ��Ӧ��Ӧ��ϡ������Cu��Ӧ��������ͭ��NO��ˮ����Ӧ���ӷ���ʽΪ��3Cu+8H++2NO3-=2Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=2Cu2++2NO��+4H2O��
��5��ȡ����Һ���������ŨNaOH��Һ�����ȣ�������ɫ��������ɫ�̼�����ζ���壮��һ��ʱ���ɫ������Ϊ����ɫ�����ձ�Ϊ���ɫ����˵���������������Ӻ�笠����ӣ���ȡ����Һ���������BaCl2��Һ������ɫ������������������ܽ⣬��˵����������������ӣ�1mol���к���6mol�ᾧˮ����ÿ�����к���6���ᾧˮ����Ħ������Ϊ392g/mol����Ļ�ѧʽΪ��NH4��2Fe��SO4��2?6H2O����NH4��2Fe��SO4��2������KMnO4��Һ��Ӧ�����ӷ���ʽΪ5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O���ʴ�Ϊ���٣�NH4��2Fe��SO4��2?6H2O����5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
���������⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ����漰��������Ų�������ʽ���Ȼ�ѧ����ʽ��Ԫ�������ɵȣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
�����Ŀ
���й��̲��漰��ѧ�仯���ǣ�������
| A������ḯʴ���� |
| B�����˳�ȥˮ�е���ɳ |
| C��ʳ����ʸ��� |
| D���ղ��ù����������ó��ֺ���ɫ�� |
������������ȷ���ǣ�������
| A�����ˮ����μ�����������FeCl3��Һ�����Ƶ�Fe��OH��3���� |
| B����ʹ��ʪ�ĵ���KI��ֽ�����ɫ������һ����C12 |
| C��ij��Һ����CCl4��CCl4������ɫ��֤��ԭ��Һ�д���I- |
| D��HCl��Һ��NaCl��Һ��ͨ�����ӵ��磬����HCl��NaCl�������ӻ����� |
�������ӷ���ʽ��ȷ���ǣ�������
| A��Fe��ϡ���ᷴӦ��2Fe+6H+�T2Fe3++3H2�� | ||||
| B��Na2O2����ˮ����O2��Na2O2+H2O�T2Na++2OH-+O2�� | ||||
C����ʯī���缫��ⱥ��MgCl2��Һ��2Cl-+2H2O
| ||||
| D��ǿ����Һ��NaClO��Fe��OH��3��Ӧ����Na2FeO4��4OH-+3ClO-+2Fe��OH��3�T2FeO42-+3Cl-+5H2O |
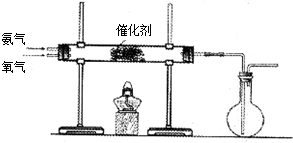
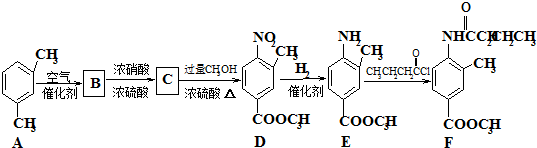
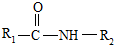 ��һ�������¿�ˮ��Ϊ
��һ�������¿�ˮ��Ϊ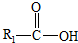 ��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��