��Ŀ����
ij����С����ʵ�����Ʊ��������������йذ���������̽����
��1����С��ͬѧ������ʯ�����Ȼ�淋Ļ������ȡ����İ�������д����С��ͬѧ�Ʊ������Ļ�ѧ����ʽ ��
��2�����ڸ����Բ����ƿ���ռ���ȡ�İ����������֤�������ռ����� ��
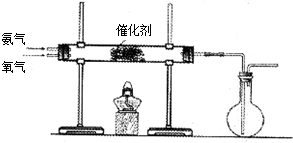
��3����С��ͬѧ�����ͼ��ʾװ��̽�������Ļ�ԭ�ԣ�
�ٰ��������Ļ�ѧ����ʽΪ ��
����ʵ��ʱͨ�백�������ʹ��죬����ƿ�л���ְ��̣������ʵĻ�ѧʽΪ ����μ������������NH4+�ķ���Ϊ ��
��1����С��ͬѧ������ʯ�����Ȼ�淋Ļ������ȡ����İ�������д����С��ͬѧ�Ʊ������Ļ�ѧ����ʽ
��2�����ڸ����Բ����ƿ���ռ���ȡ�İ����������֤�������ռ�����
��3����С��ͬѧ�����ͼ��ʾװ��̽�������Ļ�ԭ�ԣ�

�ٰ��������Ļ�ѧ����ʽΪ
����ʵ��ʱͨ�백�������ʹ��죬����ƿ�л���ְ��̣������ʵĻ�ѧʽΪ
���㣺������ȡ������
ר�⣺ʵ����
��������1��������ʯ�����Ȼ�淋Ļ������ȡ������ͬʱ�����Ȼ��ƺ�ˮ���ݴ���д��ѧ����ʽ��
��2�����ð����Ǽ�С������飻
��3������һ�������°�����������Ӧ����һ��������ˮ���ڰ��������ᷴӦ��������粒���������̣�����NH4+����ʱ��������ڼ��������·�Ӧ���ɰ���������ˮ��Һ�ʼ��ԣ���ʹʪ��ĺ�ɫʯ����ֽ������
��2�����ð����Ǽ�С������飻
��3������һ�������°�����������Ӧ����һ��������ˮ���ڰ��������ᷴӦ��������粒���������̣�����NH4+����ʱ��������ڼ��������·�Ӧ���ɰ���������ˮ��Һ�ʼ��ԣ���ʹʪ��ĺ�ɫʯ����ֽ������
���
�⣺��1��������ʯ�����Ȼ�淋Ļ������ȡ������ͬʱ�����Ȼ��ƺ�ˮ����ѧ����ʽΪCa��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O���ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��
��2�����ð����Ǽ������壬��ʹʯ��������飬���巽���ǣ���ʪ��ĺ�ɫʯ����ֽ������ƿ�ڣ���������֤�������Ѿ��ռ������ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ������ƿ�ڣ�����������˵���Գ�����
��3���ٰ��������IJ�����һ��������ˮ���ǹ�ҵ����������һ��������һ����Ӧ����ѧ����ʽΪ4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
�ڰ���������ΪNO��ˮ��������������Ӧ�������ᣬ��ͨ�백�����죬������NH4NO3���������̣�����笠����ӣ�����������ܹ��ͼӦ���ɰ��������飬���巽��Ϊ��NH4NO3ȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������笠���
�ʴ�Ϊ��NH4NO3��NH4NO3ȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������笠���
| ||
| ||
��2�����ð����Ǽ������壬��ʹʯ��������飬���巽���ǣ���ʪ��ĺ�ɫʯ����ֽ������ƿ�ڣ���������֤�������Ѿ��ռ������ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ������ƿ�ڣ�����������˵���Գ�����
��3���ٰ��������IJ�����һ��������ˮ���ǹ�ҵ����������һ��������һ����Ӧ����ѧ����ʽΪ4NH3+5O2
| ||
�ʴ�Ϊ��4NH3+5O2
| ||
�ڰ���������ΪNO��ˮ��������������Ӧ�������ᣬ��ͨ�백�����죬������NH4NO3���������̣�����笠����ӣ�����������ܹ��ͼӦ���ɰ��������飬���巽��Ϊ��NH4NO3ȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������笠���
�ʴ�Ϊ��NH4NO3��NH4NO3ȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������笠���
���������⿼���˰�����ʵ������ȡ�������ļ����Լ������Ļ�ѧ���ʣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���л�ѧ�����ʾ��ȷ���ǣ�������
A��HCl�ĵ���ʽ�� |
B��NH4Cl�ĵ���ʽ�� |
C��CCl4�ĵ���ʽ�� |
D�������ӵĽṹʾ��ͼΪ |




 ʵ������ij�Լ�ƿ�ı�ǩ��������ֻ����Լ������ͼ��ʾ���֣�
ʵ������ij�Լ�ƿ�ı�ǩ��������ֻ����Լ������ͼ��ʾ���֣�
 ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮
ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죮�ڳ����°������·������ʵ�飮