��Ŀ����
7��W��X��Y��ZΪǰ�����ڵ�Ԫ�أ�ԭ��������������Wԭ���и��ܼ��ϵĵ�������ȣ���2��δ�ɶԵ��ӣ�X��W��ͬһ���ڣ�Ҳ������δ�ɶԵ��ӣ�Y2+��X2-������ͬ�ĵ��ӹ��ͣ�Z��ԭ������Ϊ28����1��Zԭ�ӵļ۲�����Ų�ʽΪ3d84s2��
��2����ͬ���ڵ�����Ԫ�رȽϣ�Yԭ�ӵĵ�һ�����ܽϴ���ϴ�С������ԭ����Mgԭ�Ӽ۵����Ų�Ϊ3s2��Ϊȫ���ȶ�״̬�������ϵͣ�
��3��WX2�����У����ۼ��������ЦҼ����м���Wԭ�ӵ��ӻ��������Ϊsp�ӻ���WX32-����ԭ���ϵŵ��Ӷ���Ϊ0�����幹��Ϊƽ�������Σ�д����WX32-������ͬ�ռ乹�ͺͼ�����ʽ�ķ��ӻ����ӣ�SO3��NO3-��д���֣���
��4��������YX�ľ���ṹ��NaCl��ͬ��Y����λ����6�����۵���ڣ�����ڡ��������ڡ���Լ���ڡ���NaCl��ԭ������NaCl������ȣ�MgO���������ӵĵ�ɶࡢ���Ӱ뾶С����MgO�����ܴ���NaCl�ľ����ܣ���MgO�۵�ϸߣ��Ӿ����۽ṹ�ĽǶȽ��ͣ���
��5����W��Y��Z����Ԫ����ɵ�һ�ּ������ṹ�Ļ�������г����ԣ��侧����Wλ������λ�ã�Yλ�ڶ��ǣ�Zռ������λ�ã��û�����Ļ�ѧʽΪMgNi3C��������Yԭ����Χ���������Zԭ����12���������ͳ������Ͼ�������a=0.3812nm����ʽ����þ�����ܶȣ�g•cm-3��6.029g•cm-3��
���� W��X��Y��ZΪǰ�����ڵ�Ԫ�أ�ԭ��������������Wԭ���и��ܼ��ϵĵ�������ȣ���2��δ�ɶԵ��ӣ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p2����WΪCԪ�أ�X��W��ͬһ���ڣ�Ҳ������δ�ɶԵ��ӣ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p4����XΪOԪ�أ�Y2+��X2-������ͬ�ĵ��ӹ��ͣ���YΪMg��Z��ԭ������Ϊ28����ZΪNi��
��1�������������ԭ����д��
��2��Mgԭ�Ӽ۵����Ų�Ϊ3s2��Ϊȫ���ȶ�״̬��
��3��CO2�����нṹʽΪO=C=O��Ϊֱ���νṹ����Cԭ�Ӳ�ȡsp�ӻ���CO32-��̼ԭ�ӹµ��Ӷ���=$\frac{4+2-2��3}{2}$=0���۲���Ӷ���=3+0=3����CO32-������ͬ�ռ乹�ͺͼ�����ʽ�ķ��ӻ����ӣ�Ӧ��Ϊ�ȵ����壬ԭ��������ȡ��۵�������Ҳ��ȵ�����Ϊ�ȵ����壻
��4��MgO�ľ���ṹ��NaCl��ͬ���������ӻ����������������λ��Ϊ6�����ӵ��Խ�ࡢ���Ӱ뾶ԽС��������Խ������۵�Խ�ߣ�
��5��C��Mg��Ni�γ���������ṹ�Ļ����������Cԭ��λ������λ�ã�Mgԭ��λ�ڶ��ǣ�Niռ������λ�ã����ݾ�̯�����㾧����C��Mg��Niԭ����Ŀ������ȷ����ѧʽ��
�Զ���Yԭ���о�����֮�����Zԭ�Ӷ��������ģ�ÿ������Ϊ8���������ã�ÿ����Ϊ2���������ã�
��Ͼ����к���ԭ����Ŀ����ʾ�������������ٸ��ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺W��X��Y��ZΪǰ�����ڵ�Ԫ�أ�ԭ��������������Wԭ���и��ܼ��ϵĵ�������ȣ���2��δ�ɶԵ��ӣ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p2����WΪCԪ�أ�X��W��ͬһ���ڣ�Ҳ������δ�ɶԵ��ӣ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p4����XΪOԪ�أ�Y2+��X2-������ͬ�ĵ��ӹ��ͣ���YΪMg��Z��ԭ������Ϊ28����ZΪNi��
��1��Z��ԭ������Ϊ28�������������ԭ�����۵����Ų�ʽΪ3d84s2��
�ʴ�Ϊ��3d84s2��
��2��Mgԭ�Ӽ۵����Ų�Ϊ3s2��Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ�
�ʴ�Ϊ���ϴ�Mgԭ�Ӽ۵����Ų�Ϊ3s2��Ϊȫ���ȶ�״̬�������ϵͣ�
��3��CO2�����нṹʽΪO=C=O�����ЦҼ����м���Ϊֱ���νṹ����Cԭ�Ӳ�ȡsp�ӻ���CO32-��̼ԭ�ӹµ��Ӷ���=$\frac{4+2-2��3}{2}$=0���۲���Ӷ���=3+0=3��Ϊƽ�������Σ���CO32-������ͬ�ռ乹�ͺͼ�����ʽ�ķ��ӻ����ӣ�Ӧ��Ϊ�ȵ����壬ԭ��������ȡ��۵�������Ҳ��ȵ�����Ϊ�ȵ����壬�ʷ�����������ΪSO3��NO3-�ȣ�
�ʴ�Ϊ���Ҽ����м���sp�ӻ���0��ƽ�������Σ�SO3��NO3-��
��4��MgO�ľ���ṹ��NaCl��ͬ���������ӻ����������������λ��Ϊ6����NaCl������ȣ�MgO���������ӵĵ�ɶࡢ���Ӱ뾶С����MgO�����ܴ���NaCl�ľ����ܣ���MgO�۵�ϸߣ�
�ʴ�Ϊ��6�����ڣ���NaCl������ȣ�MgO���������ӵĵ�ɶࡢ���Ӱ뾶С����MgO�����ܴ���NaCl�ľ����ܣ���MgO�۵�ϸߣ�
��5��C��Mg��Ni�γ���������ṹ�Ļ����������Cԭ��λ������λ�ã�Mgԭ��λ�ڶ��ǣ�Niռ������λ�ã�����Cԭ����ĿΪ1��Mgԭ����ĿΪ8��$\frac{1}{8}$��Niԭ����ĿΪ6��$\frac{1}{2}$=3���ʻ�ѧʽΪMgNi3C��
�Զ���Yԭ���о�����֮�����Zԭ�Ӷ��������ģ�ÿ������Ϊ8���������ã�ÿ����Ϊ2���������ã��ʾ�����Yԭ����Χ���������Zԭ����$\frac{8��3}{2}$=12��
��������Ϊ$\frac{��12+59��3+12��g/mol}{6.02��1{0}^{23}mo{l}^{-1}}$���ʾ����ܶ�Ϊ$\frac{��12+59��3+12��g/mol}{6.02��1{0}^{23}mo{l}^{-1}}$�£�0.3812��10-7 cm��3=6.029g•cm-3��
�ʴ�Ϊ��MgNi3C��12��6.029g•cm-3��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ���ѧ�����ӻ���ʽ��ռ乹�͡��ȵ����塢�������������ʡ���������ȣ��Ƕ����ʽṹ����֪ʶ���ۺϿ��飬��5����ע����ݾ�̯�����м��㣬��Ŀ�������ϴ�Ϊ�״��㣮

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| A�� | ͼ1�У�A��B�Ĺ�������0.005mol���ӷ�����ת�� | |
| B�� | ͼ1���������й�����0.18gˮ | |
| C�� | ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������A | |
| D�� | ͼ1��A��B��ѧʽ�ֱ�ΪCu2O��CuO |
| A�� | ̼�⻯��������� | |
| B�� | ˮ����������鶼��10�����ӵķ��� | |
| C�� | ����ķе�ȼ���ߣ��������Һ�� | |
| D�� | ��������������ֻ��һ���⣬������Ȼ���Ҳֻ��һ�� |
 Ϊ̽��Ag+��Fe3+�����Ե�������⣬ijС��ͬѧ��������ʵ�飺
Ϊ̽��Ag+��Fe3+�����Ե�������⣬ijС��ͬѧ��������ʵ�飺��֪��������ʵ�Ksp��20�ȣ� AgCl��1.8��10-10 Ag2SO4��1.4��10-5
��1����ͬѧ��ʵ�����£�
| ��� | ���� | ���� |
| ʵ��� | ��2mL1mol/L��AgNO3��Һ���뵽 1mL1mol/L��FeSO4��Һ�� | ������ɫ����������к�ɫ������� |
| ȡ�ϲ���Һ���μ�KSCN��Һ | ��Һ��� |
�ٰ�ɫ�����Ļ�ѧʽ��Ag2SO4��
�ڼ�ͬѧ�ó�Ag+������Fe2+���������к�ɫ���壨Ag�����ɣ�����KSCN��Һ���죮
��2����ͬѧΪ̽��Ag+��Fe2+��Ӧ�ij̶ȣ�����ʵ���
a������ͼ����װ�ò�����ҩƷ�������е����ʲ����뷴Ӧ�������ֵ�ѹ��ָ��ƫ�ƣ�ƫ�Ƶķ��������������ʯī������������������һ��ʱ���ָ��ƫ�Ƽ�С��
b���������ձ�������ŨFe2��SO4��3��Һ�����ֵ�ѹ��ָ��ı仯����Ϊ��ƫ�Ƽ�С���ص���������ƫ�ƣ�
��a�м��ձ���ĵ缫��Ӧʽ��Fe2+-e-=Fe3+��
��b�е�ѹ��ָ������ƫ�ƺ���Ϊ�������������������
����ʵ��ó�Ag+��Fe2+��Ӧ�����ӷ���ʽ��Fe2++Ag+?Fe3++Ag��
��3��Ϊ��һ����֤��ͬѧ�Ľ��ۣ���ͬѧ�ֽ���������ʵ�飺
| ��� | ���� | ���� |
| ʵ��� | ��2mL2mol/LFe��NO3��3��Һ�������������Թ��� | ��������������ʧ |
| ʵ��� | ��2mL1mol/LFe2��SO4��3��Һ�������������Թ��� | �������٣�δ��ʧ |
| ʵ��� | ��2mL2mol/LFeCl3��Һ�������������Թ��� | ������ʧ |
���û�ѧ��Ӧԭ������ʵ�����V������������ͬ��ԭ����Һ�д���ƽ�⣺Fe3++Ag?Fe2++Ag+���� AgCl��Ag2SO4 Ksp�����ܽ�ȣ���С��Cl-��SO42-�������ڽ���Ag+Ũ�ȣ�����ʵ�����ʵ���������еij̶ȸ�����AgCl��Ag2SO4 Ksp�����ܽ�ȣ���С����ʹƽ�������ƶ��������ܽ⣩��
| A�� | 7 | B�� | 8 | C�� | 9 | D�� | 10 |
| A�� | ���µIJ��� | B�� | �뵼�� | C�� | ���� | D�� | ũҩ |
 ��֪��Ӧ��2CH3COCH3��l��?CH3COCH2COH��CH3��2��l����ȡ����CH3COCH3���ֱ���0���20���£������ת��������ʱ��仯�Ĺ�ϵ���ߣ�Y-t����ͼ��ʾ������˵������ȷ���ǣ�������
��֪��Ӧ��2CH3COCH3��l��?CH3COCH2COH��CH3��2��l����ȡ����CH3COCH3���ֱ���0���20���£������ת��������ʱ��仯�Ĺ�ϵ���ߣ�Y-t����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | b����20����CH3COCH3��Y-t���� | |
| B�� | ��Y=0��Y=0.113��CH3COCH2COH��CH3��2��$\frac{��n��0�棩}{��n��20�棩}$=1 | |
| C�� | �����¶ȿ����̷�Ӧ��ƽ���ʱ�䲢�����ƽ��ת���� | |
| D�� | ��Ӧ���е�20minĩ��CH3COCH3��$\frac{v��0�棩}{v��20�棩}$��1 |
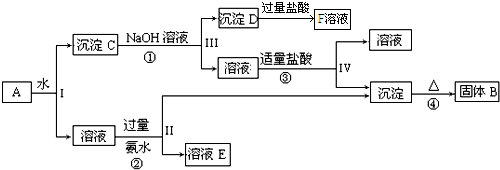
 ��
��