��Ŀ����
3�� ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��1��SO2������Fe3+��Ӧ�IJ�����Fe2+��SO42-��H+�������ӷ��ţ����μӷ�Ӧ��SO2��Fe3+�����ʵ���֮����1��2��
��2������ʵ�鷽����������ʵ������ȡ����SO2����BD��
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D��ͭ����ŨH2SO4
��3��װ��C�������dz�ȥ�����SO2����ֹ��Ⱦ������
��4����Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ���������BF������ţ���
A�������� B��ʯ���� C��©�� D���ձ� E�������� F������
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�������KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��죮
�����ۣ�����������Һ�м�����ϡ�����ữ��BaCl2��������ɫ������
�����������������Ƿ����٣�ԭ������ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
���� ��1������װ��A��Ӧ�����ӷ���ʽSO2+2Fe3++2H2O�T2Fe2++SO42-+4H+���н��
��2��ʵ������ȡ����Ҫ���Dz������㡢���ơ����ܺ����ʣ�
��3������������д̼�����ζ����Ⱦ������������������Һ���ն�������ֹ������Ⱦ��
��4������Һ�л�þ���IJ���Ϊ����������ȴ�ᾧ�����ˡ���Ȼ���������������Ͳ�������������©�����ձ�����������
��5�������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ��
��� �⣺��1�����������������ӷ�Ӧ�����ӷ���ʽΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+����Ӧ�������ӱ��������������ӣ����Ի�ԭ����Ϊ�������ӣ�����������SO42-��SO2���廹ԭFe3+��Ӧ�IJ�����Fe2+��SO42-��SO2������Fe3+��Ӧ�IJ�����Fe2+��SO42-��H+���μӷ�Ӧ��SO2��Fe3+�����ʵ���֮����1��2��
�ʴ�Ϊ��Fe2+��SO42-��H+��1��2��
��2��A���������ǿ�����ԣ��ܹ����������������������ƣ����õ������������壬��A����
B��Ũ���������ǿ���ԣ���Ũ����ӷ�������������Һ��Ũ�����ܹ���Ӧ���ɶ����������壬��B��ȷ��
C���������ڴ�����ȼ�գ������������ƣ�������ô����Ķ�������C����
D��Cu��Ũ�����ڼ������������ɶ�����������ͭ��ˮ����֪�Ʊ���������D��ȷ��
�ʴ�Ϊ��BD��
��3����������������������������д̼�����ζ��ֱ���ŷŻ���Ⱦ���������ڶ��������ܺͼӦ�����κ�ˮ�����ü�Һ����������������װ��C������Ϊ������SO2β������ֹ��Ⱦ������
�ʴ�Ϊ����ȥ�����SO2����ֹ��Ⱦ������
��4������Һ�л�þ���IJ���Ϊ����������ȴ�ᾧ�����ˡ���Ȼ���������������Ͳ�������������©�����ձ�������������������һϵ�в�����û���õ���������ʯ������������
�ʴ�Ϊ��BF��
��5�����������л�ԭ�ԣ����������ǿ�����ԣ������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ�����Է����ٲ�������
�ʴ�Ϊ�������٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
���� ���⿼������������ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ������漰�����Ի�ԭ��ǿ���Ƚϡ�����ʵ�鷽������������۵�֪ʶ����ȷ����Ũ��������ʡ���������ļ��鷽����֪ʶΪ�����ؼ���

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| A�� | 3d3��ʾ3d�ܼ�����3����� | |
| B�� | ns�ܼ���ԭ�ӹ��ͼ����������ͼ��ʾ | |
| C�� | 1s�����Ƴ����Σ���ʾ������ԭ�Ӻ���Բ���˶� | |
| D�� | ������ͼ�ĺڵ��ܶ�Խ��˵������ռ������Խ�� |

| A�� | �ӳɷ�Ӧ | B�� | ��ȥ��Ӧ | C�� | ȡ����Ӧ | D�� | ��ԭ��Ӧ |
| A�� | C3H8 | B�� | C4H10 | C�� | C5H12 | D�� | C6H14 |
 �ڻ�ҩ��ըʱ�������ֻ�ѧ��Ӧ��������Ҫ��ѧ��Ӧ����ʽΪ��
�ڻ�ҩ��ըʱ�������ֻ�ѧ��Ӧ��������Ҫ��ѧ��Ӧ����ʽΪ�� ��
��
 ��
�� ���õ���ʽ��ʾFM2���γɹ���
���õ���ʽ��ʾFM2���γɹ��� ��
�� ��
��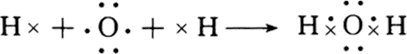 ��
��