��Ŀ����
 X��Y��Z���ֳ���Ԫ�صĵ��ʣ��ס��������ֳ����Ļ�����������ͼת����ϵ���ش��������⣺
X��Y��Z���ֳ���Ԫ�صĵ��ʣ��ס��������ֳ����Ļ�����������ͼת����ϵ���ش��������⣺��1����X��̬ԭ����Χ�����Ų�ʽΪ3s2�������ɵڶ���������Ԫ�ص�ԭ�ӹ��ɵ���̬��������ӣ�Y��̬ԭ�ӵĹ����ʾʽΪ
��2����Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����������YΪ�ӷ���Һ�����ʡ���Ϊ��ɫ������ˮ�����壮��ZΪ
��3����X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����Ϊ���д��Եĺ�ɫ���壬��X���Ӧ�Ļ�ѧ����ʽΪ��
���㣺������ƶ�,ԭ�Ӻ�������Ų�
ר�⣺�ƶ���
��������1����X��̬ԭ����Χ�����Ų�ʽΪ3s2�������ɵڶ���������Ԫ�ص�ԭ�ӹ��ɵ���̬��������ӣ���XΪMg����ΪCO2����ΪMgO��YΪC��
��2��Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����n-1=2����n=3����X�ļ۵�����Ϊ7��XΪCl2����Ϊ��ɫ������ˮ�����壬��YΪH2O����ΪHCl��ZΪH2��������YΪ�ӷ���Һ�����ʣ�YΪBr2��
��3��X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����XΪAl����Ϊ���д��Եĺ�ɫ���壬��ΪFe3O4����ΪAl2O3��YΪFe��
��2��Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����n-1=2����n=3����X�ļ۵�����Ϊ7��XΪCl2����Ϊ��ɫ������ˮ�����壬��YΪH2O����ΪHCl��ZΪH2��������YΪ�ӷ���Һ�����ʣ�YΪBr2��
��3��X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����XΪAl����Ϊ���д��Եĺ�ɫ���壬��ΪFe3O4����ΪAl2O3��YΪFe��
���
�⣺��1����X��̬ԭ����Χ�����Ų�ʽΪ3s2�������ɵڶ���������Ԫ�ص�ԭ�ӹ��ɵ���̬��������ӣ���XΪMg����ΪCO2����ΪMgO��YΪC����CO2�ĵ���ʽΪ ��Y��ԭ������Ϊ6�����̬ԭ�ӵĹ����ʾʽΪ
��Y��ԭ������Ϊ6�����̬ԭ�ӵĹ����ʾʽΪ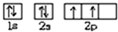 ��
��
�ʴ�Ϊ�� ��
�� ��
��
��2��Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����n-1=2����n=3����X�ļ۵�����Ϊ7��XΪCl2����Ϊ��ɫ������ˮ�����壬��YΪH2O����ΪHCl��ZΪH2��������YΪ�ӷ���Һ�����ʣ�YΪBr2��Br��ԭ������Ϊ35�����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d64s24p5��
�ʴ�Ϊ��H2��[Ar]3d64s24p5��
��3��X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����XΪAl����Ϊ���д��Եĺ�ɫ���壬��ΪFe3O4����ΪAl2O3��YΪFe����X���Ӧ�Ļ�ѧ����ʽΪ8Al+3Fe3O4�T9Fe+4Al2O3��Fe��ԭ������Ϊ26���������Ӻ�����24�����ӣ���Y2+���ӵĵ����Ų�ʽΪ[Ar]3d6��
�ʴ�Ϊ��8Al+3Fe3O4�T9Fe+4Al2O3��[Ar]3d6��
 ��Y��ԭ������Ϊ6�����̬ԭ�ӵĹ����ʾʽΪ
��Y��ԭ������Ϊ6�����̬ԭ�ӵĹ����ʾʽΪ ��
���ʴ�Ϊ��
 ��
�� ��
����2��Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����n-1=2����n=3����X�ļ۵�����Ϊ7��XΪCl2����Ϊ��ɫ������ˮ�����壬��YΪH2O����ΪHCl��ZΪH2��������YΪ�ӷ���Һ�����ʣ�YΪBr2��Br��ԭ������Ϊ35�����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d64s24p5��
�ʴ�Ϊ��H2��[Ar]3d64s24p5��
��3��X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����XΪAl����Ϊ���д��Եĺ�ɫ���壬��ΪFe3O4����ΪAl2O3��YΪFe����X���Ӧ�Ļ�ѧ����ʽΪ8Al+3Fe3O4�T9Fe+4Al2O3��Fe��ԭ������Ϊ26���������Ӻ�����24�����ӣ���Y2+���ӵĵ����Ų�ʽΪ[Ar]3d6��
�ʴ�Ϊ��8Al+3Fe3O4�T9Fe+4Al2O3��[Ar]3d6��
���������⿼��������ƶϣ�Ϊ��Ƶ���㣬���յ����Ų������ʵ����ʼ�ת����ϵ���ƶ�����Ϊ���Ĺؼ��������û���Ӧ��ԭ�ӽṹ�����ʵĿ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
Ǧ�����ڷŵ�ʱ��ԭ��ص����ã����ʱ����ص����ã�Ǧ�����ڷŵ�ͳ��ʱ�����Ļ�ѧ��ӦΪ��Pb+PbO2+2H2SO4
2PbSO4+2H2O������������ȷ���ǣ�������
| �ŵ� |
| ��� |
| A���ŵ�ʱ��������Ӧʽ��Pb+SO42--2e-�TPbSO4 |
| B���ŵ�һ��ʱ���������Χ��Һ���������� |
| C�����ʱ��������Ӧʽ��PbSO4+2H2O-2e-�TPbO2+4H++SO42- |
| D�����ʱ��Ҫʹ2mol PbSO4��ΪPb��PbO2��Ҫͨ��4mol���� |
���е���ʽ��д��ȷ���ǣ�������
| A��Na��Cl | B����N����N�� |
| C��H��H | D��H��Cl |
 ȫ�������ܵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ����ͼ��ʾ������H+�������Dz���������Ӧ����ͨ������Ĥ�����ƶ�ʹ�Ҳ���Һ���ֵ����ԣ������й�˵������ȷ���ǣ�������
ȫ�������ܵ�������ò�ͬ��̬���ӶԵ�������ԭ��Ӧ��ʵ�ֻ�ѧ�ܺ͵����ת����װ�ã���ԭ����ͼ��ʾ������H+�������Dz���������Ӧ����ͨ������Ĥ�����ƶ�ʹ�Ҳ���Һ���ֵ����ԣ������й�˵������ȷ���ǣ�������| A���ŵ�ʱ�������Һ���ɻƱ�������缫��ӦʽΪ��VO2++e-+2H+�TVO2++H2O |
| B�����ʱ��ת�Ƶĵ�����Ϊ3.01��1023���������Һ��n��H+���ı仯��Ϊ1.0mol |
| C�����ʱ��H+����۶����ƶ����Ҳ� |
| D���������У��Ҳ���Һ��ɫ������ɫ��Ϊ��ɫ |
