��Ŀ����
A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ����������������֪��
��Aԭ�Ӻ���ֻ��1�����ӣ���Bԭ�������������Ǵ�����������2������Cԭ�������������ȴ�����������4������Dԭ�ӵĴ�����������������������8������E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ����F��Cͬ���壮�û�ѧ���Ż�ѧ����ش��������⣺
��1���õ���ʽ��ʾ��������A2C2 ��DCA ��
��2���õ���ʽ��ʾD��F�γɻ�����Ĺ���
��3���á���ѧʽ���͡������Ƚϣ�A��C��A��F�γɻ�������ȶ��� ���е�ĸߵ� �е�ߵ͵�ԭ��
��4��д��E���ռӦ�����ӷ���ʽ
��5����AԪ�صĵ�����CԪ�صĵ��ʿ����Ƴɵ�أ������װ��KOHŨ��Һ���ö�Ľ������Ե缫����KOH��Һ�������������Ƶķ�ֹ���ĸ�Ĥ����a��ͨ��A�ĵ��ʣ�b��ͨ��C�ĵ��ʣ�a���ĵ缫��Ӧʽ�� ��b���ĵ缫��Ӧʽ�� ������ΪH2SO4���������Һ����a���ĵ缫��Ӧʽ�� ��b���ĵ缫��Ӧʽ�� ��
��Aԭ�Ӻ���ֻ��1�����ӣ���Bԭ�������������Ǵ�����������2������Cԭ�������������ȴ�����������4������Dԭ�ӵĴ�����������������������8������E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ����F��Cͬ���壮�û�ѧ���Ż�ѧ����ش��������⣺
��1���õ���ʽ��ʾ��������A2C2
��2���õ���ʽ��ʾD��F�γɻ�����Ĺ���
��3���á���ѧʽ���͡������Ƚϣ�A��C��A��F�γɻ�������ȶ���
��4��д��E���ռӦ�����ӷ���ʽ
��5����AԪ�صĵ�����CԪ�صĵ��ʿ����Ƴɵ�أ������װ��KOHŨ��Һ���ö�Ľ������Ե缫����KOH��Һ�������������Ƶķ�ֹ���ĸ�Ĥ����a��ͨ��A�ĵ��ʣ�b��ͨ��C�ĵ��ʣ�a���ĵ缫��Ӧʽ��
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
��������Aԭ�Ӻ���ֻ��1�����ӣ���A���⣻
��Bԭ�������������Ǵ�����������2������BΪ̼��
��Cԭ�������������ȴ�����������4������CΪ����
��Dԭ�ӵĴ�����������������������8������DΪ�ƣ�
��E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ����EΪ����
��F��Cͬ���壬��FΪ�ݴ˽���С�⼴�ɣ�
��Bԭ�������������Ǵ�����������2������BΪ̼��
��Cԭ�������������ȴ�����������4������CΪ����
��Dԭ�ӵĴ�����������������������8������DΪ�ƣ�
��E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ����EΪ����
��F��Cͬ���壬��FΪ�ݴ˽���С�⼴�ɣ�
���
�⣺��1����A2C2��H2O2���ǹ��ۻ��������ʽΪ�� ��DCA��NaOH�������ӻ��������ʽΪ
��DCA��NaOH�������ӻ��������ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��2��Na2S��2����ԭ�Ӻ�1����ԭ��ͨ�����Ӽ��γɵģ��γɹ���Ϊ ��
��
�ʴ�Ϊ�� ��
��
��3��Ԫ�صķǽ�����Խǿ���γɵ��⻯��Խ�ȶ������Ӽ����ʹ���ʵ��ۡ��е����ߣ�
�ʴ𰸣�H2O��H2S��H2O��H2S��H2O���Ӽ���������
��4�����ܹ���Ӧ����ƫ�����κ�������2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��5����������ȼ�ϵ�أ���������������Ӧ�������ڸ����ŵ����ɵ����������������������ˮ���缫��ӦʽΪ2H2-4e-+4OH-=4H2O������������ԭ��Ӧ�������������ŵ磬�缫��ӦʽO2+2H2O+4e-=4OH-������ΪH2SO4���������Һ����a���ĵ缫��ӦʽΪ��2H2-4e-=4H+��������ӦʽΪ��O2+4e-+4H+=2H2O���ʴ�Ϊ��2H2-4e-+4OH-=4H2O��O2+4e-+2H2O=4OH-��2H2-4e-=4H+��O2+4e-+4H+=2H2O��
 ��DCA��NaOH�������ӻ��������ʽΪ
��DCA��NaOH�������ӻ��������ʽΪ ��
���ʴ�Ϊ��
 ��
�� ��
����2��Na2S��2����ԭ�Ӻ�1����ԭ��ͨ�����Ӽ��γɵģ��γɹ���Ϊ
 ��
���ʴ�Ϊ��
 ��
����3��Ԫ�صķǽ�����Խǿ���γɵ��⻯��Խ�ȶ������Ӽ����ʹ���ʵ��ۡ��е����ߣ�
�ʴ𰸣�H2O��H2S��H2O��H2S��H2O���Ӽ���������
��4�����ܹ���Ӧ����ƫ�����κ�������2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��5����������ȼ�ϵ�أ���������������Ӧ�������ڸ����ŵ����ɵ����������������������ˮ���缫��ӦʽΪ2H2-4e-+4OH-=4H2O������������ԭ��Ӧ�������������ŵ磬�缫��ӦʽO2+2H2O+4e-=4OH-������ΪH2SO4���������Һ����a���ĵ缫��ӦʽΪ��2H2-4e-=4H+��������ӦʽΪ��O2+4e-+4H+=2H2O���ʴ�Ϊ��2H2-4e-+4OH-=4H2O��O2+4e-+2H2O=4OH-��2H2-4e-=4H+��O2+4e-+4H+=2H2O��
���������⿼���˳���Ԫ�ص��ƶϣ���ɴ��⣬��������ԭ�ӽṹ��֪ʶ���У�Ҫ��ͬѧ����������1��20��Ԫ�ص�ԭ�ӽṹ���Ա����Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
�ڸ��������£����мӵ�������ڻ�ѧ��Ӧ���ܱ���ȫ���ĵ��ǣ�������
A����50 mL 8 mol?L-1
| ||||||
B����״���£�1 g��ƬͶ��20 mL 18.4 mol?L-1��
| ||||||
C����100 mL 3 mol?L-1��
| ||||||
D����5��107 Pa��500�������ý���������£���28g
|
��ͼ��Ԫ�����ڱ�ǰ�����ڵ�һ���֣������й�R��W��X��Y��Z����Ԫ�ص������У���ȷ���ǣ�������


| A����ѹ������Ԫ�صĵ�����Z���ʵķе���� |
| B��Y��Z�������ӵ��Ӳ�ṹ����Rԭ�ӵ���ͬ |
| C��W���⻯�ﻹԭ��С��Y���⻯�� |
| D��YԪ�صķǽ����Ա�WԪ�صķǽ�����ǿ |
Ԫ��X��Y��Zԭ������֮��Ϊ36��X��Y��ͬһ���ڣ�X+��Z2-������ͬ�ĺ�����Ӳ�ṹ�������Ʋⲻ��ȷ���ǣ�������
| A��ͬ����Ԫ����X�Ľ�������ǿ |
| B��ͬ����Ԫ����Y����ۺ������������ǿ |
| C��ԭ�Ӱ뾶X��Y�����Ӱ뾶X+��Z2- |
| D��ͬ��Ԫ����Z���⻯���ȶ������ |
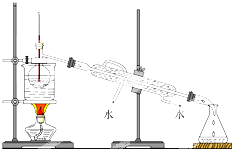
 �Ϸ���ijУ��ѧ��ȤС��Ϊ̽������Ũ���ᷴӦ���������ͼ��ʾװ�ý���ʵ�飮
�Ϸ���ijУ��ѧ��ȤС��Ϊ̽������Ũ���ᷴӦ���������ͼ��ʾװ�ý���ʵ�飮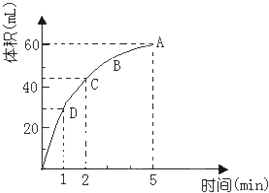 Ϊ���о�MnO2��˫��ˮ��H2O2���ķ�Ӧ���ʣ�ijѧ������������MnO2��ĩ��50mL�ܶ�Ϊ1.1g/cm3��˫��ˮ��Һ�У�ͨ��ʵ��ⶨ���ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ������ͼ�ش��������⣺
Ϊ���о�MnO2��˫��ˮ��H2O2���ķ�Ӧ���ʣ�ijѧ������������MnO2��ĩ��50mL�ܶ�Ϊ1.1g/cm3��˫��ˮ��Һ�У�ͨ��ʵ��ⶨ���ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ������ͼ�ش��������⣺