��Ŀ����
1�������أ������ĺ��������Ϊ��ɫ��״���壬�����ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156��157�棬���ȶ��Բ�������Ǹ�Ч�Ŀ�űҩ����֪�����ѷе�Ϊ35�棬����������ȡ�����صķ���֮һ������ȡԭ��Ϊ�����ģ���Ҫ�����ѽ�ȡ�������ͽ�ȡ�������ѽ�ȡ������Ҫ����Ϊ��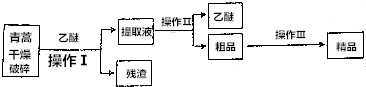
��ش��������⣺
��1����������и��������Ŀ�����������������ѵĽӴ��������������صĽ����ʣ�
��2����������Ҫ�IJ���������Ҫ�У��ձ�����������©����Ϊ���ٲ�����Ľ��У���ò��ó��˵ķ����������������������
��3�����������Ҫ���̿�����B������ĸ����
A����ˮ�ܽ⣬����Ũ������ȴ�ᾧ
B����95%�����ѣ�Ũ�����ᾧ������
C���������ѽ�����ȡ��Һ��
���� �����أ������ĺ��������Ϊ��ɫ��״���壬��ˮ�м������ܣ������ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��������и������飬�������ѽ�ȡͨ�����˵õ���ȡҺ�Ͳ�������ȡҺͨ������õ����Ѻʹ�Ʒ����Ʒͨ����95%�����ѣ�Ũ�����ᾧ�����˵õ���Ʒ��
��1������Ӵ����������Ӧ���ʺ��ܽ����ʣ�
��2���������Ƿ�������Һ��IJ���Ϊ����װ�ã��ݴ�ѡ����������Ϊ���ٲ�����Ľ��п������ó���װ�ã���������ͨ��������ȥ���ѣ�
��3�������أ������ĺ��������Ϊ��ɫ��״���壬�����ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156��157�棬���ȶ��Բ���ѷе�Ϊ35����ڳ�ȥ��
��� �⣺��1����������и��������Ŀ�����������������ѵĽӴ��������������صĽ����ʣ�
�ʴ�Ϊ���������������ѵĽӴ��������������صĽ����ʣ�
��2�����������ڷ�������Һ�壬����ù��˵ķ������룬ѡ��������Ϊ�ձ�����������©����Ϊ���ٲ�����Ľ��п������ó���װ�ã������������ڷ������ѣ���������ķ�����
�ʴ�Ϊ����������©�������ˣ�����
��3�����������Ϣ������֪�������أ������ĺ��������Ϊ��ɫ��״���壬�����ڱ�ͪ���ȷºͱ��У��ڼ״����Ҵ������ѡ�ʯ�����п��ܽ⣬��ˮ�м������ܣ��۵�Ϊ156��157�棬���ȶ��Բ���ѷе�Ϊ35����ڳ�ȥ��Aѡ�������ز�����ˮ��Cѡ���Һ�õ��Ļ��ǻ��Һ��B����95%�����ѣ�Ũ�����ᾧ���������ᴿ��ʵ�鷽����
�ʴ�Ϊ��B��
���� ���⿼���Ϊ�ۺϣ��漰���ʵķ��롢�ᴿ�Լ����ʵ�������ؼ��㣬����ѧ���ķ�����ʵ�������Ŀ��飬��Ŀ��Ϊ������ע��������ʵ������Լ����������ʵ�����������Ŀ���еȣ�

| A�� | SiH4��CH4�ȶ� | B�� | S2-�뾶��Cl-��С | ||
| C�� | 7834Se��8034Se��Ϊͬ�������� | D�� | �ǽ����ԣ�O��N��P��Si |
| A�� | MgO��H2SO4��Na2O��CaCl2 | B�� | MnO2��HNO3��KOH��K2CO3 | ||
| C�� | SO2��NaHSO4��Ca��OH��2��KCl | D�� | CH3OH��CH3COOH��C2H5OH��CH4 |
| A�� | ǿ������Һ�У�K+��Al3+��Cl-��SO42- | |
| B�� | ���д���NH4+����Һ��Mg2+��S2-��OH-��I- | |
| C�� | ͨ������NO2����Һ��K+��Na+��SO32-��AlO2- | |
| D�� | ǿ������Һ�У�Na+��Fe3+��NO3-��SO42- |
| A�� | NaHBΪǿ����� | B�� | NaHB��Һ�У�c��Na+����c��HB-��+2c��B2-�� | ||
| C�� | H2BΪ������� | D�� | HB-�ĵ���̶�С��HB-��ˮ��̶� |
| A�� | NH4+��Al3+��SO42-��NO3- | B�� | K+��Na+��ClO-��NH4+ | ||
| C�� | K+��NH4+��MnO4-��SO42- | D�� | Na+��K+��NO3-��HSO3- |
| A�� | SO2��C�����ʯ�� | B�� | CO2��H2 | C�� | NaCl��HCl | D�� | MgCl2��KCl |
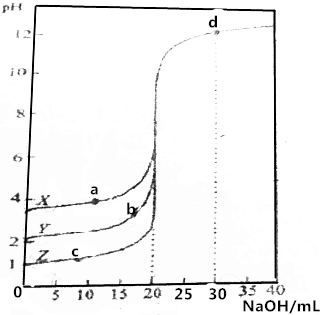 ��0.1000mol•L-1��NaOH��Һ�ֱ�ζ�0.1000mol•L-1��20.00mLX��Y��Z��������Һ����ҺpH�����NaOH���֮��Ĺ�ϵ��ͼ��ʾ������˵��������ǣ�������
��0.1000mol•L-1��NaOH��Һ�ֱ�ζ�0.1000mol•L-1��20.00mLX��Y��Z��������Һ����ҺpH�����NaOH���֮��Ĺ�ϵ��ͼ��ʾ������˵��������ǣ�������| A�� | ZΪһԪǿ�� | |
| B�� | d���c��OH-��Ϊ0.02000mol•L-1 | |
| C�� | a��b��c��b��������ӵ����ʵ���Ũ����� | |
| D�� | X��YΪһԪ���ᣬ������볣����Ka��x����Ka��Y�� |
 �ĵ⻯����SnI4����һ�ֳȺ�ɫ�ᾧ���۵�Ϊ144.5�棬�е�Ϊ364�棬��������ˮ�����ڴ��������ȷµȣ���ˮ��ˮ�⣬�����������Լ����л��ϳ��Լ���ʵ�����Ʊ��ĵ⻯������Ҫ�������£�
�ĵ⻯����SnI4����һ�ֳȺ�ɫ�ᾧ���۵�Ϊ144.5�棬�е�Ϊ364�棬��������ˮ�����ڴ��������ȷµȣ���ˮ��ˮ�⣬�����������Լ����л��ϳ��Լ���ʵ�����Ʊ��ĵ⻯������Ҫ�������£�