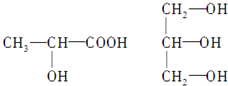��Ŀ����
��ҵ���û�ͭ��ұ��ͭ����¯���ۺ����õ�һ�ֹ����������£�

��1��ұ�������еõ�Cu2O��Cu�Ļ�����Ϊ����ͭ�����������A1�ڸ��������»�Ϸ�Ӧ�ɵô�ͭ����Ӧ��ѧ����ʽΪ ����ͭ����ʱӦ����ͭ������ֱ����Դ�� �������� ���õ����Ƚϸߵľ�ͭ��
��2����ͳ��ͭ�ķ�����Ҫ�ǻ���ͭ������Ҫ��ӦΪ��
��2CuFeS2+4O2
Cu2S+3SO2+2FeO
��2Cu2S+3O2
2Cu2O+2SO2
��2Cu2O+Cu2S
6Cu+SO2��
ÿ����1mol Cu�������� mol O2����Ӧ���е��������� ��
��3����ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3���������̻ش��������⣺
�ټ�������NaClO��Һ��Ŀ���� �������ӷ���ʽ��ʾ����
�ڳ�ȥAl3+�����ӷ���ʽ�� ��
��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO���ṩ���Լ��У�ϡ���ᡢϡ���ᡢKSCN��Һ��KMnO4��Һ��NaOH��Һ����ˮ����ѡ�Լ��� ��ʵ����ƣ� ��

��1��ұ�������еõ�Cu2O��Cu�Ļ�����Ϊ����ͭ�����������A1�ڸ��������»�Ϸ�Ӧ�ɵô�ͭ����Ӧ��ѧ����ʽΪ
��2����ͳ��ͭ�ķ�����Ҫ�ǻ���ͭ������Ҫ��ӦΪ��
��2CuFeS2+4O2
| ||
��2Cu2S+3O2
| ||
��2Cu2O+Cu2S
| ||
ÿ����1mol Cu��������
��3����ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3���������̻ش��������⣺
�ټ�������NaClO��Һ��Ŀ����
�ڳ�ȥAl3+�����ӷ���ʽ��
��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO���ṩ���Լ��У�ϡ���ᡢϡ���ᡢKSCN��Һ��KMnO4��Һ��NaOH��Һ����ˮ����ѡ�Լ���
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������
��������1��������Ϣ������A1�ڸ��������º�Cu2O��Cu�Ļ���ﷴӦ�ɵô�ͭ����д����ʽ����⾫��ͭʱ�������Ǵ�ͭ����������ͭ�����ݵ��صĹ���ԭ�����ش�
��2���ɷ�Ӧ��֪������6Cu��6CuFeS2��15O2��������ԭ��Ӧ�У����ϼ۽���Ԫ�����ڵķ�Ӧ�������������Դ˷�����
��3���ٴ������ƾ��������ԣ��ܽ���ԭ�Ե�����������
�������ӿ��Ժ�����ǿ����Һ��Ӧ����ƫ�����Σ��������ʵ��������ش�
�۸����������ӵ�������ȷ��ѡ����Լ����������ӵ��������ӷ�Ӧ�����ӽ��м��鼴�ɣ�
��2���ɷ�Ӧ��֪������6Cu��6CuFeS2��15O2��������ԭ��Ӧ�У����ϼ۽���Ԫ�����ڵķ�Ӧ�������������Դ˷�����
��3���ٴ������ƾ��������ԣ��ܽ���ԭ�Ե�����������
�������ӿ��Ժ�����ǿ����Һ��Ӧ����ƫ�����Σ��������ʵ��������ش�
�۸����������ӵ�������ȷ��ѡ����Լ����������ӵ��������ӷ�Ӧ�����ӽ��м��鼴�ɣ�
���
�⣺��1��������Ϣ������A1�ڸ��������º�Cu2O��Cu�Ļ���ﷴӦ�ɵô�ͭ������ʽΪ��3Cu2O+2Al
Al2O3+6Cu����⾫��ͭʱ�������Ǵ�ͭ�����Դ�ͭ�͵�Դ��������������������ͭ���ڸõ缫���������ǽ���ͭ���ʴ�Ϊ��3Cu2O+2Al
Al2O3+6Cu����������
��2���ɷ�Ӧ��2CuFeS2+4O2
Cu2S+3SO2+2FeO
��2Cu2S+3O2
2Cu2O+2SO2
��2Cu2O+Cu2S
6Cu+SO2��
��֪�����ڹ�ϵʽ��6Cu��6CuFeS2��15O2������ÿ����1mol Cu��������������2.5mol��������ԭ��Ӧ2Cu2O+Cu2S
6Cu+SO2���У����ϼ۽��͵�CuԪ�����ڵķ�Ӧ��Cu2O��Cu2S�����������ʴ�Ϊ��2.5��Cu2O��Cu2S��
��3����ͭ������¯������Fe2O3��FeO��SiO2��Al2O3���Ʊ�Fe2O3��ԭ�����ȼ������ᣬ�ڹ��ˣ����Եõ��Ȼ������Ȼ��������Ȼ����Ļ��Һ�������������˳�������Һ�м������������Һ���������Կ��Խ���������ȫ������Ϊ�����ӣ��ټ����������ƺ���ˣ����Եõ�����������������֮���ȷֽ�õ���������
�ټ�������NaClO��Һ��Ŀ���ǽ�������������Ϊ�����ӣ���2Fe2++ClO-+2H+=2Fe3++Cl-+H2O���ʴ�Ϊ��2Fe2++ClO-+2H+=2Fe3++Cl-+H2O��
�������ӿ��Ժ�����ǿ����Һ��Ӧ����ƫ�����Σ����ˣ�����ʵ�������Ӻ������ӵķ��룬����ȥ�����ӣ���ȥAl3+�����ӷ���ʽ�ǣ�Al3++4OH-=[Al��OH��4]-���ʴ�Ϊ��Al3++4OH-=[Al��OH��4]-��
������������������ϡ�����У�����������ʹ���������ɫ�����Խ�¯������ϡ�����У�ȡ��Һ�����еμӸ��������Һ�������ɫ��ȥ��֤��
�����������ӣ�֤��¯���к���FeO��������ϡ���ᣬ��Ϊ����Ҳ��ʹ���������ɫ�������������ӵļ��飬
�ʴ�Ϊ��ϡ���ᡢKMnO4��Һ����¯������ϡ�����У�ȡ��Һ�����еμӸ��������Һ�������ɫ��ȥ��֤�������������ӣ�֤��¯���к���FeO��
| ||
| ||
��2���ɷ�Ӧ��2CuFeS2+4O2
| ||
��2Cu2S+3O2
| ||
��2Cu2O+Cu2S
| ||
��֪�����ڹ�ϵʽ��6Cu��6CuFeS2��15O2������ÿ����1mol Cu��������������2.5mol��������ԭ��Ӧ2Cu2O+Cu2S
| ||
��3����ͭ������¯������Fe2O3��FeO��SiO2��Al2O3���Ʊ�Fe2O3��ԭ�����ȼ������ᣬ�ڹ��ˣ����Եõ��Ȼ������Ȼ��������Ȼ����Ļ��Һ�������������˳�������Һ�м������������Һ���������Կ��Խ���������ȫ������Ϊ�����ӣ��ټ����������ƺ���ˣ����Եõ�����������������֮���ȷֽ�õ���������
�ټ�������NaClO��Һ��Ŀ���ǽ�������������Ϊ�����ӣ���2Fe2++ClO-+2H+=2Fe3++Cl-+H2O���ʴ�Ϊ��2Fe2++ClO-+2H+=2Fe3++Cl-+H2O��
�������ӿ��Ժ�����ǿ����Һ��Ӧ����ƫ�����Σ����ˣ�����ʵ�������Ӻ������ӵķ��룬����ȥ�����ӣ���ȥAl3+�����ӷ���ʽ�ǣ�Al3++4OH-=[Al��OH��4]-���ʴ�Ϊ��Al3++4OH-=[Al��OH��4]-��
������������������ϡ�����У�����������ʹ���������ɫ�����Խ�¯������ϡ�����У�ȡ��Һ�����еμӸ��������Һ�������ɫ��ȥ��֤��
�����������ӣ�֤��¯���к���FeO��������ϡ���ᣬ��Ϊ����Ҳ��ʹ���������ɫ�������������ӵļ��飬
�ʴ�Ϊ��ϡ���ᡢKMnO4��Һ����¯������ϡ�����У�ȡ��Һ�����еμӸ��������Һ�������ɫ��ȥ��֤�������������ӣ�֤��¯���к���FeO��
������������һ����ѧ�������̷�ʽ�������Ʊ�ʵ�鷽������⣬ע�����ʵķ�����ᴿ������ķ����ǹؼ�������ѧ�������ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
���жԵ縺�Ե����ⲻ��ȷ���ǣ�������
| A���縺������Ϊ�涨��һ�������ֵ�����Ǿ��Ա� |
| B��Ԫ�ص縺�ԵĴ�С��ӳ��Ԫ�ضԼ��ϵ��������Ĵ�С |
| C��Ԫ�صĵ縺��Խ����Ԫ�صķǽ�����Խǿ |
| D��Ԫ�صĵ縺����Ԫ�ع��е����ʣ���ԭ�ӽṹ�� |
�����������ʵ��ó��Ľ�����ȷ���ǣ�������
| A����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Һһ����̼������Һ |
| B���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һһ����������Һ |
| C��Al��Fe��Cu��Ӧ��������������ᷴӦ�����κ�ˮ�����ֽ������������Ϊ���������� |
| D����ij��Һ�еμ���ˮ���ٵμ�KSCN��Һ����Һ��Ѫ��ɫ������Һ�в�һ����Fe2+ |
����˵����ȷ���ǣ�������
| A��100mL1mol/L Al2��SO4��3��Һ�У�Al3+��Ϊ0.2��6.02��1023 |
| B��0.1molп��100mL1mol/L ��ϡ�����ַ�Ӧ�����������ķ�����Ϊ0.1��6.02��1023 |
| C��C60��C70�Ļ���ﹲ12g����������̼ԭ����Ϊ6.02��1023 |
| D��1mol����-CH3����1mol��������OH-��������������Ϊ10��6.02��1023 |
���и���̬ԭ�Ӻ����ӵĵ����Ų�ʽ������ǣ�������
| A��Ca2+��1s22s22p63s23p6 |
| B��F-��1s22s23p6 |
| C��S2-��1s22s22p63s23p4 |
| D��Ar��1s22s22p63s23p6 |
�����йر�����ȷ���ǣ�������
| A�������ӵĵ����Ų�ʽ��1s22s22p63s23p4 |
B��H2O�ĵ���ʽ��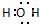 |
C��Nԭ���������ӵĹ����ʾʽ��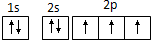 |
D��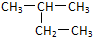 �����ƣ�2-�һ����� �����ƣ�2-�һ����� |
���з��ӻ������У�����ԭ�Ӻ��йµ��ӶԵ��ǣ�������
| A��H3O+ |
| B��SiH4 |
| C��PH3 |
| D��SO42- |
 �����ϳ�·����ͼ��ʾ�����ַ�Ӧ�Լ������������ʡ�ԣ���
�����ϳ�·����ͼ��ʾ�����ַ�Ӧ�Լ������������ʡ�ԣ���