��Ŀ����
1��ʵ������һ����ɫ���壬���ܺ�������李������ⰱ�е�һ�ֻ����֣�Ϊȷ����ɷ֣��ֳ�ȡ24.70g�İ�ɫ������������ˮ�У�Ȼ��μ�4mol•L-1������������Һ50.00mL�����ȣ����¶�����β��ֽ⣩��ַ�Ӧʹ����ȫ���ݳ�����ð����ڱ���µ����Ϊ2.24L�������б�����ȷ���ǣ�������| A�� | ��ɫ����һ��ֻ��������� | |
| B�� | ������������Һ�����������ɰ��������ӦΪ6.72L������� | |
| C�� | �������������������ɫ�����У�NH4��2SO4��NH4HSO4�����ʵ���֮��Ϊ1��2 | |
| D�� | ��ij��ɫ������ȷֽ⣬�����������岻��ʹʪ��ĺ�ɫʯ����ֽ��������ù�����һ��������� |
���� A������뷴Ӧ�ģ�NH4��2SO4��NH4HSO4���ֱ�Ϊxmol��ymol��
��NH4��2SO4+2NaOH=2NH3��+Na2SO4+2H2O
1 2 2
x 2x 2x
NH4HSO4+2NaOH=NH3��+Na2SO4+2H2O
1 2 1
y 2y y
n��NH3��=$\frac{2.24L}{22.4L/mol}$=0.1mol����2x+y=0.1�ڣ�n��NaOH��=2x+2y=0.05L��4mol/L�ڣ������٢ڣ��ݴ˽��з�������ijɷ֣�
B����A��n��NH4HSO4��=0.1mol��m����NH4��2SO4��=24.7g-0.1mol��115g/mol=13.2g����n����NH4��2SO4��=$\frac{13.2g}{132g/mol}$=0.1mol�����ݵ�Ԫ���غ�n��NH3��=2n����NH4��2SO4��+n��NH4HSO4��=2��0.1mol+0.1mol=0.3mol������V=nVm���м��㣻
C�����AB�����ݷ�����
D������μ��������¿������ɵ���������
��� �⣺A������뷴Ӧ�ģ�NH4��2SO4��NH4HSO4���ֱ�Ϊxmol��ymol��
��NH4��2SO4+2NaOH=2NH3��+Na2SO4+2H2O
1 2 2
x 2x 2x
NH4HSO4+2NaOH=NH3��+Na2SO4+2H2O
1 2 1
y 2y y
n��NH3��=$\frac{2.24L}{22.4L/mol}$=0.1mol����2x+y=0.1�ڣ�n��NaOH��=2x+2y=0.05L��4mol/L�ڣ������٢ڣ����x=0��y=0.1���ʹ����п϶���NH4HSO4����m��NH4HSO4��=0.1mol��115g/mol=11.5g��11.5g��24.70g���ʺ� ��NH4��2SO4��NH4HSO4����A����
B����A��n��NH4HSO4��=0.1mol��m����NH4��2SO4��=24.7g-0.1mol��115g/mol=13.2g����n����NH4��2SO4��=$\frac{13.2g}{132g/mol}$=0.1mol�����ݵ�Ԫ���غ�n��NH3��=2n����NH4��2SO4��+n��NH4HSO4��=2��0.1mol+0.1mol=0.3mol������V=nVm=0.3mol��22.4L/mol=6.72L����B��ȷ��
C����A��B��n��NH4HSO4��=0.1mol��n����NH4��2SO4��=$\frac{13.2g}{132g/mol}$=0.1mol���ʣ�NH4��2SO4��NH4HSO4�����ʵ���֮��Ϊ1��1����C����
D������μ������������ɵ�������������ʹʪ��ĺ�ɫʯ����ֽ��������D����
��ѡB��
���� ���⿼���˻������ɵ��ƶϣ���ɴ��⣬���Խ������ṩ����Ϣ���з����������ԭ���غ���м��㣬�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | һ����ȩ������1��̼ԭ�ӡ�2����ԭ�ӡ�1����ԭ�ӹ��ɵ� | |
| B�� | ��ȩ��̼���⡢������Ԫ�ص�������Ϊ1��2��1 | |
| C�� | ��ȩ��̼���⡢������Ԫ����ɵ� | |
| D�� | ��ȩ����Ԫ�ص���������Ϊ53.3% |
| ѡ�� | ʵ����������� | ���� |
| A | ȡ������������Һ�������м�������������ˮ���ڵμ�KSCN��Һ����Һ��Ѫ��ɫ | ����Һ�к���Fe3+ |
| B | �����£���Ũ�Ⱦ�Ϊ0.1mol•L-1��BaCl2��CaCl2�����Һ�еμ�Na2SO4��Һ�����ְ�ɫ������ | Ksp��Ba2SO4����Ksp��Ca2SO4�� |
| C | �����£���FeCl3��Һ�еμ�����KI��Һ���ٵμӼ��ε�����Һ����Һ����ɫ | Fe3+�������Ա�I2��ǿ |
| D | �����£���PH��ֽ��ã�0.1mol•L-1 Na2SO3��Һ��PHԼΪ10��0.1mol•L-1 NaHSO3��Һ��PHԼΪ5 | HSO3-���H+��������SO32-��ǿ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ˮ�м����������������ú��ϲ���ɫ��dz���²���ɫ��Ϊ�Ϻ�ɫ | |
| B�� | ���ȵ�ͭ˿��������ȼ�գ��������ػ�ɫ������ | |
| C�� | ±�ص��ʣ�X2����ˮ��Ӧ������X2+H2O=HX+HXO��ʾ | |
| D�� | �廯����Һ�м����������Ƶ���ˮ���ټ����������Ȼ�̼�����ú��ϲ���ɫ��dz���²���ɫ��Ϊ�Ⱥ�ɫ |
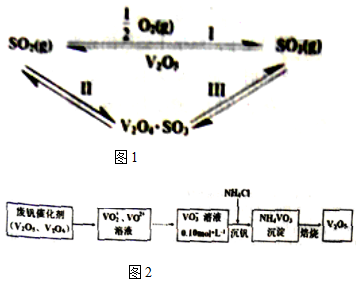
��1�����з�����1mol��ѧ������ʱ��Ҫ���յ��������������
| ��ѧ�� | S=O��SO2�� | S=O��SO3�� | O=O��O2�� |
| ����/kJ | 535 | 472 | 496 |
��2���ӷϷ������л���V2O5�Ĺ�����ͼ2��
��VO2+���ӿ��ɷ�ͬ��̬�ļ���������ȫˮ��õ�����ˮ�ⷴӦ�����ӷ���ʽV4++H2O=VO2++2H+��
�ڡ�������ʱΪʹ��Ԫ�صij����ʴﵽ98%������Ӧ������Һ�е�c��NH4+��Ϊ0.8mol•L-1[25�棬Ksp��NH4VO3��=1.6��10-3����Һ����仯���Բ���]��
��3��������������������ͬʱ����SO2��NOx����ã�NH4��2SO4��ϡ��Һ��
�������Һϡ��Һ���ټ���������NH4��2SO4���壬$\frac{c��N{{H}_{4}}^{+}��}{c��S{{O}_{4}}^{2-}��}$��������������䡱��С������
�ڳ����£�0.05mol/L��NH4��2SO4��Һ��pH=a����$\frac{c��N{{H}_{4}}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$=1.7��10��9-a�����ú�a�Ĵ���ʽ��ʾ��NH3•H2O��Kb=1.7��10-5��
| A�� | ��ȥ����Cu���е�����Mg�ۺ�Al�ۣ���ϡ�������� | |
| B�� | �������ͺ�ú�ͣ�������ȡ�ķ��� | |
| C�� | ��������غ��Ȼ��ƹ���Ļ��������ܽ⡢���˵ķ��� | |
| D�� | �������������Ļ������ͨ�����ȵ�����ͭ���Գ�ȥ���е����� |
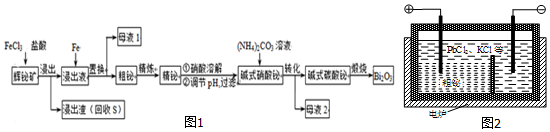
 ��
��