��Ŀ����
 ��0.1mol��þ�������������100mL 2mol/LH2SO4��Һ�У�Ȼ���ٵμ�1mol/L NaOH��Һ����ش�
��0.1mol��þ�������������100mL 2mol/LH2SO4��Һ�У�Ȼ���ٵμ�1mol/L NaOH��Һ����ش���1�����ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ�����V�仯����ͼ��ʾ����V1=140mLʱ���������ĩ��n��Mg��=
��2���μ�NaOH��Һ
��3�����������Ϊ0.1mol������Mg�۵����ʵ�������Ϊa����100mL 2mol/L�������ܽ�˻������ټ���480mL 1mol/L��NaOH��Һ�����ó�������Al��OH��3�������������a��ȡֵ��Χ�ǣ�
��4�����μ�NaOH��Һ��V2mlʱ��ֹͣ�μ�NaOH��Һ����ʼ����Һ��ͨ������CO2����д��������Ӧ�����ӷ���ʽ
���㣺�йػ���ﷴӦ�ļ���
ר�⣺������
��������1������ͼ���֪���ڵμ�NaOH��Һ�����V1=140mL�����У�û�г������ɣ�˵��������ʣ�࣬�μӵ�NaOH�����к�ʣ�����ᣬV1=160mLʱ��ʣ���H2SO4��μӵ�NaOHǡ����ȫ��Ӧ����Һ��MgSO4��Al2��SO4��3��Na2SO4���Һ����MgSO4Ϊxmol��Al2��SO4��3Ϊymol�������غ��з�������n��Mg����
���μ�NaOH��Һ�����V2ʱ��Al��OH��3��ȫ�ܽ⣬������Mg��OH��2����Һ��Na2SO4��NaAlO2���Һ�������غ㣬��n��NaOH��=2n��Na2SO4��+n��NaAlO2��=2n��H2SO4��+n��Al�����ݴ����n��NaOH������������NaOH��Һ�����
��2�����������ʱ����Һ������ΪNa2SO4�������غ���n��NaOH��=2n��Na2SO4��=2n��H2SO4������������NaOH��Һ�����
��3����Ӧ��Ļ����Һ���ټ���480mL 1mol/L��NaOH��Һ�����ó�������Al��OH��3������Ϊ�����ơ�ƫ�����ƣ�����Ϊ����������a��1�������������غ㣬Ӧ����n��NaOH����2n��Na2SO4��+n��NaAlO2����������Ԫ���غ���x��ʾ��n��NaAlO2�����ݴ�ȷ��a��ȡֵ��Χ��
��4�����μ�NaOH��Һ��V2mLʱ��ֹͣ�μ�NaOH��Һ����ʱ��Һ������ΪNa2SO4��NaAlO2����ʼ����Һ��ͨ������CO2������������������̼�����ƣ�
���μ�NaOH��Һ�����V2ʱ��Al��OH��3��ȫ�ܽ⣬������Mg��OH��2����Һ��Na2SO4��NaAlO2���Һ�������غ㣬��n��NaOH��=2n��Na2SO4��+n��NaAlO2��=2n��H2SO4��+n��Al�����ݴ����n��NaOH������������NaOH��Һ�����
��2�����������ʱ����Һ������ΪNa2SO4�������غ���n��NaOH��=2n��Na2SO4��=2n��H2SO4������������NaOH��Һ�����
��3����Ӧ��Ļ����Һ���ټ���480mL 1mol/L��NaOH��Һ�����ó�������Al��OH��3������Ϊ�����ơ�ƫ�����ƣ�����Ϊ����������a��1�������������غ㣬Ӧ����n��NaOH����2n��Na2SO4��+n��NaAlO2����������Ԫ���غ���x��ʾ��n��NaAlO2�����ݴ�ȷ��a��ȡֵ��Χ��
��4�����μ�NaOH��Һ��V2mLʱ��ֹͣ�μ�NaOH��Һ����ʱ��Һ������ΪNa2SO4��NaAlO2����ʼ����Һ��ͨ������CO2������������������̼�����ƣ�
���
�⣺��1����V1=140mLʱ����ʱ����Һ��MgSO4��Al2��SO4��3��Na2SO4���Һ��
��Na+�����غ��֪��n��Na2SO4��=
n��NaOH��=
��0.14L��1mol/L=0.07mol
��MgSO4Ϊxmol��Al2��SO4��3Ϊymol����
����Mgԭ�ӡ�Alԭ���غ��У�x+2y=0.1
����SO42-�����غ��У�x+3y=0.1��2-0.07
�������̣���ã�x=0.04��y=0.03
���Խ�����ĩ��n��Mg��=0.04mol��n��Al��=2y=2��0.03mol=0.06mol��
�μ�NaOH��Һ�����V2ʱ����Һ��Na2SO4��NaAlO2���Һ�������غ��У�
n��NaOH��=2n��Na2SO4��+n��NaAlO2��=2n��H2SO4��+n��Al��=2��0.1L��2mol/L+0.06mol=0.46mol��
���ԣ�V2=
=0.46L=460mL��
�ʴ�Ϊ��0.04��460��
��2������Һ��Mg2+��Al3+ǡ�ó�����ȫʱ����ʱ��Һ��Na2SO4��Һ������SO42-���Ӻ�Na+�����غ��У�n��Na+��=2n��Na2SO4��=2n��H2SO4��=2��0.1L��2mol/L=0.4mol�����ԣ�V��NaOH��=
=0.4L=400mL��
�ʴ�Ϊ��400��
��3������Ϊ����������a��1��Al�����ʵ���Ϊ0.1��1-a��mol����Ӧ��Ļ����Һ���ټ���480mL 1mol/L��NaOH��Һ�����ó�������Al��OH��3������Ϊ�����ơ�ƫ�����ƣ�������Ԫ���غ��֪n��NaAlO2��=0.1��1-a��mol�������������غ㣬Ӧ����n��NaOH����2n��Na2SO4��+n��NaAlO2������0.48��1��2��0.1��2+0.1��1-a�������a��0.2����0.2��a��1��
�ʴ�Ϊ��0.2��a��1��
��4�����μ�NaOH��Һ��V2mLʱ��ֹͣ�μ�NaOH��Һ����ʱ��Һ������ΪNa2SO4��NaAlO2����ʼ����Һ��ͨ������CO2������������������̼�����ƣ���Ӧ���ӷ���ʽΪ��CO2+2H2O+AlO2-=Al��OH��3��+HCO3-��
�ʴ�Ϊ��CO2+2H2O+AlO2-=Al��OH��3��+HCO3-��
��Na+�����غ��֪��n��Na2SO4��=
| 1 |
| 2 |
| 1 |
| 2 |
��MgSO4Ϊxmol��Al2��SO4��3Ϊymol����
����Mgԭ�ӡ�Alԭ���غ��У�x+2y=0.1
����SO42-�����غ��У�x+3y=0.1��2-0.07
�������̣���ã�x=0.04��y=0.03
���Խ�����ĩ��n��Mg��=0.04mol��n��Al��=2y=2��0.03mol=0.06mol��
�μ�NaOH��Һ�����V2ʱ����Һ��Na2SO4��NaAlO2���Һ�������غ��У�
n��NaOH��=2n��Na2SO4��+n��NaAlO2��=2n��H2SO4��+n��Al��=2��0.1L��2mol/L+0.06mol=0.46mol��
���ԣ�V2=
| 0.46mol |
| 1mol/L |
�ʴ�Ϊ��0.04��460��
��2������Һ��Mg2+��Al3+ǡ�ó�����ȫʱ����ʱ��Һ��Na2SO4��Һ������SO42-���Ӻ�Na+�����غ��У�n��Na+��=2n��Na2SO4��=2n��H2SO4��=2��0.1L��2mol/L=0.4mol�����ԣ�V��NaOH��=
| 0.4mol |
| 1mol/L |
�ʴ�Ϊ��400��
��3������Ϊ����������a��1��Al�����ʵ���Ϊ0.1��1-a��mol����Ӧ��Ļ����Һ���ټ���480mL 1mol/L��NaOH��Һ�����ó�������Al��OH��3������Ϊ�����ơ�ƫ�����ƣ�������Ԫ���غ��֪n��NaAlO2��=0.1��1-a��mol�������������غ㣬Ӧ����n��NaOH����2n��Na2SO4��+n��NaAlO2������0.48��1��2��0.1��2+0.1��1-a�������a��0.2����0.2��a��1��
�ʴ�Ϊ��0.2��a��1��
��4�����μ�NaOH��Һ��V2mLʱ��ֹͣ�μ�NaOH��Һ����ʱ��Һ������ΪNa2SO4��NaAlO2����ʼ����Һ��ͨ������CO2������������������̼�����ƣ���Ӧ���ӷ���ʽΪ��CO2+2H2O+AlO2-=Al��OH��3��+HCO3-��
�ʴ�Ϊ��CO2+2H2O+AlO2-=Al��OH��3��+HCO3-��
������������ͼ����ʽ����������㣬���ÿһ��ͼ�����Ļ�ѧ��Ӧ��֪���յ�����ĺ��弰��Һ�����ʵijɷ֣�����غ�˼����н���Ѷ��еȣ�

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
�����Ŀ
���ܱ������У����ڷ�Ӧ2SO2��g��+O2��g��?2SO3��g����SO2��O2��ʼʱ�ֱ�Ϊ2.0mol��1.0mol����ƽ��ʱ��SO2��ת����Ϊ80%������SO3��ʼ���з�Ӧ������ͬ���¶��£���ʹƽ��ʱ���ɷֵİٷֺ�����ǰ����ͬ��������ʼʱSO3�����ʵ�������ת����Ϊ��������
| A��1.0 mol�� 10% |
| B��2.0 mol�� 80% |
| C��2.0 mol�� 40% |
| D��2.0 mol�� 20% |
��13.6g�Ȼ�п��Ʒ�����л�����һ���Ȼ��������Ʒ����ˮ�����������������Һ���ɵó���29.5g������Ʒ�п��ܺ���������һ���Ȼ��������
| A���Ȼ��� | B���Ȼ��� |
| C���Ȼ�þ | D���Ȼ��� |
��������̬����ɵĻ�����壬������̼Ԫ�غ���Ԫ�ص�����֮��Ϊ24��3�������������ɼ�������ȿ����ǣ�������
| A��CH4��C3H4�������Ϊ1��1 |
| B��C2H2��C2H6�������Ϊ3��1 |
| C��C2H4��C2H6�������Ϊ2��1 |
| D��C2H2 ��C2H4�������Ϊ2��3 |
�ҹ�����������������Ƶ����PM2.5���γ�������������ף�PM2.5��Ҫ��ָ��������
| A��������̼ | B��һ����̼ |
| C���������� | D������������� |
������ʵ��װ����ɶ�Ӧ��ʵ�飬���ܴﵽʵ��Ŀ���ǣ�������
| A | B | C | D | |
| װ�� | 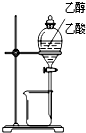 | 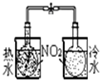 | 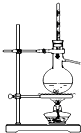 |  |
| ʵ�� | �����Ҵ������� | ֤���¶ȶԻ�ѧƽ���Ӱ�� | ����е����ϴ�Ļ���Һ������ | ��ȡ���ռ����� |
| A��A | B��B | C��C | D��D |
�����ռNaOH�����Ƿ���д��Na2CO3�������·������е��ǣ�������
| A�����Ⱥ�۲��Ƿ���������� |
| B������ˮ��μ�ϡ�Ȼ�����Һ�۲��Ƿ��г������� |
| C������ˮ�������Һ�Ƿ��Լ��� |
| D������ˮ��ͨ��CO2�۲��Ƿ���NaHCO3���� |
��һ�ܱ�������ʢ��V��Cl2��H2�Ļ�����������������³�ַ�Ӧ���ٻָ���ԭ״��ʱ�����������ΪV��������Щ����ͨ��NaOH��Һʱȫ�����գ��ɴ��ж�ԭ���������Cl2��H2������ȿ����ǣ�������
| A������1��1 |
| B����1��1 |
| C������1��1 |
| D��С�ڻ����1��1 |
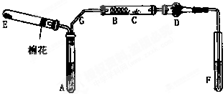 ��ͼ��ij��ѧ��ȤС����Ƶ��Ҵ���������ʵ��װ�ã�ͼ�м�������������̨���Թܼо�δ��������ͼ��A��Ϊ��ˮ�Ҵ����е�78�棩��B��Ϊ�Ƴ�����״��ϸͭ˿����˿��C��Ϊ��ˮCuSO4��ĩ��D��Ϊ��ʯ�ң�F��Ϊ���Ƶļ���Cu��OH��2����Һ��
��ͼ��ij��ѧ��ȤС����Ƶ��Ҵ���������ʵ��װ�ã�ͼ�м�������������̨���Թܼо�δ��������ͼ��A��Ϊ��ˮ�Ҵ����е�78�棩��B��Ϊ�Ƴ�����״��ϸͭ˿����˿��C��Ϊ��ˮCuSO4��ĩ��D��Ϊ��ʯ�ң�F��Ϊ���Ƶļ���Cu��OH��2����Һ��