��Ŀ����
2����һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K+��Mg2+��A13+��Ba2+��Fe2+��NO3-��SO42-��Cl-��I-��HCO3-��ȡ����Һʵ�����£�| ʵ�鲽�� | ʵ������ |
| ��1��ȡ��������Һ���Ӽ��μ��� | ��Һ���ɫ |
| ��2��ȡ��������Һ����Ũ������CuƬ�� ŨH2SO4������ | ����ɫ����������������������Ա�ɺ���ɫ |
| ��3��ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| ��4��ȡ��3�����ϲ���Һ����AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����HNO3 |
| ��5��ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
��1����Һ�п϶����ڵ�������NO3-��SO42- Mg2+��Al3+����Һ�п϶������ڵ�������I-��Ba2+��Fe2+��HCO3-�����ܴ��ڵ�������K+��Cl-
��2����μ�����Һ���Ƿ��п��ܴ��ڵ������� ��д�������������ۣ�ȡ����ԭ��Һ�����������ᱵ��Һ�����˺�����Һ�м���������Һ���а�ɫ�������ɣ��ټ�ϡ���ᣬ��ɫ�������ܽ⣬˵����Cl-����֮��û�У�
���� ������ɫ����Һ������Fe2+���ɣ�1��֪��Һ�����ԣ�����HCO3-���ɣ�2��֪��NO���ɣ�ԭ��Һ�к�NO3-����һ������Fe2+��I-�����л�ԭ�ԣ����ɣ�3��֪��SO42-���ڣ���ԭ��Һ����Ba2+���ɣ�4������ȷ���Ƿ�Cl-����3������Cl-���ɢ�֪��Mg2+��Al3+��
��� �⣺������ɫ����Һ������Fe2+��
���ݣ�1��ȡ��������Һ���Ӽ��μ�����Һ����Һ�Ժ�ɫ��˵����Һ��ʾ���ԣ�����HCO3-�����ڣ�
���ݣ�2��ȡ��������Һ����Ũ������CuƬ��Ũ���ᣬ��������ɫ������������壨NO�����������Ա�ɺ���ɫ��������������˵����Һ�к���NO3-����һ��������I-��
���ݣ�3��ȡ��������Һ����BaCl2��Һ���а�ɫ�������ɣ�����SO42-��
���ݣ�4��ȡ��3���е��ϲ���ҹ����AgNO3�����ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���ᣬ��3������Һ�к���Cl-�����ڸ��ݣ�3���м������Ȼ���������Cl-������ȷ��ԭ��Һ���Ƿ����Cl-��
���ݣ�5��ȡ��������Һ������NaOH��Һ�а�ɫ�������ɣ���NaOH����ʱ�����������ܽ⣬��ԭ��Һ�к���Mg2+��Al3+��
��1�����Ͽ�֪����Һ��һ�����ڵ������ǣ�NO3-��SO42- Mg2+��Al3+����Һ�п϶������ڵ������ǣ�I-��Ba2+��Fe2+��HCO3-������ȷ�����У�K+��Cl-��
�ʴ�Ϊ��NO3-��SO42- Mg2+��Al3+��I-��Ba2+��Fe2+��HCO3-��K+��Cl-��
��2����Һ�еĿ��ܴ��ڵ���������Cl-�����鷽����Ϊ��ȡ����ԭ��Һ�����������ᱵ��Һ�����˺�����Һ�м���������Һ���а�ɫ�������ɣ��ټ�ϡ���ᣬ��ɫ�������ܽ⣬˵����Cl-����֮��û�У�
�ʴ�Ϊ��ȡ����ԭ��Һ�����������ᱵ��Һ�����˺�����Һ�м���������Һ���а�ɫ�������ɣ��ټ�ϡ���ᣬ��ɫ�������ܽ⣬˵����Cl-����֮��û�У�
���� ���⿼�鳣�����ӵļ���ͼ����Ǹ߿��еij������ͣ��ۺ���ǿ������������ѧ����������������ʵ���������������Ԫ�ػ����������ǹؼ���ע�����ճ������ӵļ��飬�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| X | Y | ||
| Z | W |
| A�� | �����£�����Ԫ�ص����У�ȫ���ǹ��� | |
| B�� | Z����������Y�������ӵ��Ӳ�ṹ��ͬ | |
| C�� | X����̬�⻯���Y����̬�⻯���ȶ� | |
| D�� | WԪ��ԭ�Ӱ뾶��ZԪ��ԭ�Ӱ뾶�� |
| A�� | NH4+K+NO3-Cl- | B�� | NO3-CO32-K+Na+ | ||
| C�� | K+Na+ Cl-SO42- | D�� | Mg2+Cu2+SO42-Cl- |
| A�� | CH2O | B�� | C2H4O | C�� | C2H4O2 | D�� | C3H8O |
| A�� | �����ӵĽṹ������̼ԭ�ӵ����ӷ�ʽ�ǵ�˫��������ɵĻ�״ | |
| B�� | ���к���̼̼˫�������Ա�����ϩ�� | |
| C�� | ��������6��̼̼��ѧ����ȫ��ͬ | |
| D�� | ����������ˮ�����������Һ��Ӧ��ʹ������ɫ |
| A�� | �Ȼ��� | B�� | ���� | C�� | NH4Cl�ĵ���ʽ  | D�� | ���� |
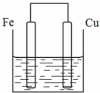 ��������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�����ͼ��װ�ã������¾����跴Ӧ��������Һ������䣩��
��������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�����ͼ��װ�ã������¾����跴Ӧ��������Һ������䣩��