��Ŀ����
18������β���������ࡢCO��SO2��NO�����ʣ��dz��п�������ȾԴ֮һ�������ķ���֮һ������������������װһ����ת��������Pt��Pd�Ͻ����������������ص���ʹCO��NO��Ӧ�����ɿɲ��������̬����ѭ���������壬����ʹ���ͳ��ȼ�ռ�SO2��ת������1��д��NO��COͨ����ת����������Ӧ�Ļ�ѧ����ʽ��2CO+2NO$\frac{\underline{\;����\;}}{\;}$N2+2CO2��
��2����ת������ȱ������һ���̶�������˿�������ȣ���ԭ���Ƿ�Ӧ��������N2�⣬�����������ӿ�����ȵ�CO2��SO3��
��3�����Ƴ��п�����ȾԴ�ķ�����bc����ѡ���ţ���
a��ֲ������ b����������Դc��ʹ�õ綯�� d��������������e��ʹ����Ǧ���ͣ�
���� ��1��һ��������һ����̼�ڴ��������·�Ӧ���ɵ����Ͷ�����̼��
��2����SO2�ɴ�����ΪSO3��SO3�ڿ��������γ�H2SO4��
��3����������Դ��ʹ�õ綯�����ٻ�ʯȼ�ϵ�ȼ�տ��Լ�����ȾԴ���̻����С������������ǡ�ʹ����Ǧ���͡�ֲ�����ֿ��Լ���һЩ������Ⱦ��������ȾԴû�й�ϵ��
��� �⣺��1���������֪��һ��������һ����̼�ڴ��������·�Ӧ���ɵ����Ͷ�����̼����ѧ����ʽ��2CO+2NO$\frac{\underline{\;����\;}}{\;}$N2+2CO2��
�ʴ�Ϊ��2CO+2NO$\frac{\underline{\;����\;}}{\;}$N2+2CO2��
��2��һ��������һ����̼�ڴ��������·�Ӧ���ɵ����Ͷ�����̼��������̼Ϊ�������壬�����ڡ���ת�������ٽ���SO2��ת�������ɵ�SO3���࣬SO3+H2O=H2SO4������˿�������ȣ�
�ʴ�Ϊ����Ӧ��������N2�⣬�����������ӿ�����ȵ�CO2��SO3��
��3����������Դ��ʹ�õ綯���ܹ����ٻ�ʯȼ�ϵ�ȼ�տ��Լ�����ȾԴ���̻����С������������ǡ�ʹ����Ǧ���͡�ֲ�����ֿ��Լ���һЩ������Ⱦ��������ȾԴû�й�ϵ��
��ѡ��bc��
���� ���⿼���˻�����Ⱦ����������ȷ���ʵ����ʼ������γɹ����ǽ���ؼ�����Ŀ�ѶȲ���

 ij��ѧС���Ի������Ʊ�����ϩ����ͼ1����
ij��ѧС���Ի������Ʊ�����ϩ����ͼ1������֪��
 $��_{85��}^{ŨH_{2}SO_{4}}$
$��_{85��}^{ŨH_{2}SO_{4}}$ +H2O
+H2O| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�������������
���Թ�C���ڱ�ˮԡ�е�Ŀ����ʹ����ϩҺ�������ٻӷ���
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȣ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ㣨���ϻ��£�����Һ����c ������ĸ��ϴ�ӣ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ����ͼ2װ��������ȴˮ��g�ڽ��루����ĸ��������ʱҪ������ʯ�ң�Ŀ�����������ɵ�ˮ����ֹˮ�����滷��ϩһ��������
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����c������ĸ����
a������ʱ��70�濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������bc������ĸ����
a�������Ը��������Һ b���ý����� c���ⶨ�е㣮
| ��������� | ���� | |
| A | 100mL H2O | ��ˮ�������c��H+��•c��OH-������ |
| B | 0.01molK2O | ��Һ��$\frac{c��HC{{O}_{3}}^{-}��}{c��O{H}^{-}��}$ ���� |
| C | 50mL 1mol/LH2SO4 | ��Ӧ������c��Na+��=c��SO42-�� |
| D | 0.1molKHSO4���� | ��Ӧ��������ҺpH=7 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ȥSO2�е�����HCl��ͨ�뱥��NaHSO3��Һ��ϴ��ƿ��������ռ����� | |
| B�� | FeCl2��Һ�л���FeCl3������������۳�ַ�Ӧ����� | |
| C�� | Na2CO3�����л�������NaHCO3����������NaOH��Һ | |
| D�� | ��ȥSiO2�е�����Al2O3����������ϡ�����ַ�Ӧ����� |
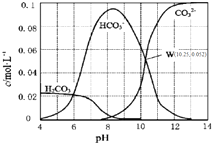 25��ʱ��0.1mol•L-1̼������Һ��ͨ��HCl���壬��̼���ӵ�Ũ����pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
25��ʱ��0.1mol•L-1̼������Һ��ͨ��HCl���壬��̼���ӵ�Ũ����pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��pH=7.0ʱ����Һ�к�̼����ֻ��CO32-��HCO3- | |
| B�� | ��Na2CO3��Һ��ͨ��HCl���壬��������CO2���� | |
| C�� | H2CO3��Ka2=1.0��10-10.25 | |
| D�� | ��100 mL 0.1 mol•L-1̼������Һ�еμ���������ҺpH=4.0������CO2����224 mL |
 ��
��