��Ŀ����
1��H��һ�����ϣ�������ͼ����Ʒ����ϳɣ�
��֪������һ�������£��л���������ת����ϵ��

����A�͵����ʵ�����HCl�ڲ�ͬ�������·����ӳɷ�Ӧ���ȿ�������ֻ����һ������B��Ҳ�������ɺ�����������F��
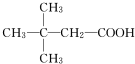
��1��D�Ľṹ��ʽΪCH3CH2CHO��
��2����A��B�Ļ�ѧ��Ӧ����ʽ��CH3CH=CH2+HCl$\stackrel{һ������}{��}$CH3CH2CH2Cl��
��3��F��G�Ļ�ѧ��Ӧ����Ϊȡ����Ӧ��G������Ϊ2-������
��4��E+G��H�Ļ�ѧ��Ӧ����ʽCH3CH2COOH+CH3CH��OH��CH3$��_{��}^{Ũ����}$CH3CH2COOCH��CH3��2+H2O��
��5��H�ж���ͬ���칹�壬���к���һ���Ȼ�������һ�ȴ��������ֵ��ǣ�
 �����ýṹ��ʽ��ʾ��
�����ýṹ��ʽ��ʾ��
���� ��ת����ϵ��֪��E�����Ȼ���G����-OH����E��G������ͬ��̼ԭ����Ŀ����E��G����������Ӧ����H����H�ķ���ʽ��֪��EΪCH3CH2COOH��F�к���2��������GΪCH3CH��OH��CH3�����ƿɵã�FΪCH3CHClCH3��DΪCH3CH2CHO��CΪCH3CH2CH2OH��BΪCH3CH2CH2Cl��AΪCH3CH=CH2���Դ˽����⣮
��� �⣺��1��������������֪��D�Ľṹ��ʽΪCH3CH2CHO��
�ʴ�Ϊ��CH3CH2CHO��
��2����A��B�Ļ�ѧ��Ӧ����ʽ�ǣ�CH3CH=CH2+HCl$\stackrel{һ������}{��}$CH3CH2CH2Cl��
�ʴ�Ϊ��CH3CH=CH2+HCl$\stackrel{һ������}{��}$CH3CH2CH2Cl��
��3��F��G��CH3CHClCH3����ȡ����Ӧ����CH3CH��OH��CH3��������Ϊ2-������
�ʴ�Ϊ��ȡ����Ӧ��2-������
��4��E+G��H�Ļ�ѧ��Ӧ����ʽΪ��CH3CH2COOH+CH3CH��OH��CH3$��_{��}^{Ũ����}$CH3CH2COOCH��CH3��2+H2O��
�ʴ�Ϊ��CH3CH2COOH+CH3CH��OH��CH3$��_{��}^{Ũ����}$CH3CH2COOCH��CH3��2+H2O��
��5��H�ж���ͬ���칹�壬���к���һ���Ȼ�������һ�ȴ��������ֵ��� ��
��
�ʴ�Ϊ�� ��
��
���� �����ۺϿ����л�����ƶϣ�Ϊ�߿��������ͣ����ؿ����л�������ʡ�ͬ���칹�塢�л���Ӧ���͵ȣ��ѶȲ������չ����ŵ�����Խת���ǹؼ���ע�����H�ķ���ʽ�������Ʒ������ƶϣ�

| A�� | һ����Ag+ | B�� | һ����SO42- | C�� | ����Ag+��SO42- | D�� | ����Ag+��SO42- |

����˵��������ǣ�������
| A�� | ����������ʽ��������Һ��������ӦΪ2SO42--2e-�TS2O82- | |
| B�� | S2O82-����ǿ�����ԣ�H2S2O8�Ƕ�Ԫǿ�� | |
| C�� | ��ѹ�����Ŀ����Ϊ�˼���H2O2�ֽ⣬����õ�����һ��ֿ�ѭ������ | |
| D�� | ����ҺAת��Ϊ��ҺB������������ԭ��Ӧ |
| A�� | CuSO4 | B�� | H2SO4 | C�� | H2O | D�� | SO2 |
| A�� | H2SO4 | B�� | CH3COOH | C�� | ��NH4��2 SO4 | D�� | NaOH |
| A�� | Na+ | B�� | Al3+ | C�� | Fe2+ | D�� | Fe3+ |
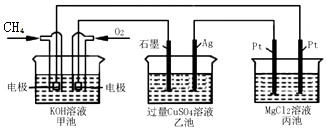
| A�� | �׳��ǵ���ת��Ϊ��ѧ�ܵ�װ�ã��ҡ������ǻ�ѧ��ת�����ܵ�װ�� | |
| B�� | �׳��������ĵ缫��Ӧʽ��O2+4e-+4H+=2H2O | |
| C�� | ��Ӧ�����У��ҳص�pH��С | |
| D�� | �׳�������O2����������������������������ͬ�����µı�ֵΪ1��2 |