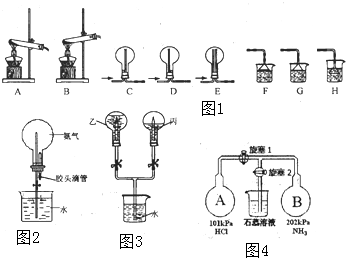
��Ŀ����
2��ʵ��������NaOH��������1.0mol•L-1��NaOH��Һ480mL����1��������Һʱ��һ����Է�Ϊ���¼������裺�ٳ��� �ڼ��� ���ܽ� �ܵ�תҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ������ȷ�IJ���˳��Ϊ�ڢ٢ۢ�ݢޢߢܣ���ʵ������õ�����������ƽ��ҩ�ס����������ձ�����ͷ�ιܡ�����500mL����ƿ��
��2��ijͬѧ������һ������NaOH���壬��������ƽ�����ձ�����������ƽƽ����״̬��ͼ�� �ձ���ʵ������Ϊ27.4g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�20.0g NaOH��

��3��ʹ������ƿǰ������е�һ�������Dz�©��
��4�������ƹ����У���������������ȷ�ģ����в���������Ũ��ƫ�ߵ��Ǣܢݣ���Ӱ����Ǣۣ�����ţ���
��û��ϴ���ձ��Ͳ����� ��ת����Һʱ������������������ƿ���� ������ƿ�����������������ˮ �ܶ���ʱ���ӿ̶��� ��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������ ���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�
��5�����ڵμ�����ˮʱ�����������˿̶��ߣ�Ӧ��δ�����ʵ��ʧ�ܣ�ϴ������ƿ�������ƣ�
���� ��1����������һ�����ʵ���Ũ�ȵ�ʵ�鲽������ѡ����ʵ�������
��2��������ƽʹ��ԭ����������=��������+��������������m=CVM�������ʵ�������
��3������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
��4�������������������ʵ����ʵ���Ũ�Ⱥ���Һ�������Ӱ�죬����C=n/V������������
��5�����ڵμ�����ˮʱ�����������˿̶��ߣ�Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�
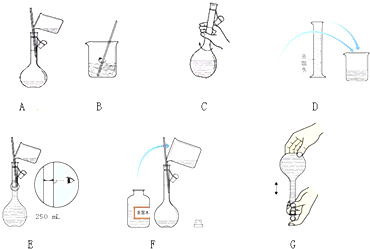
��� �⣺��1����������һ�����ʵ���Ũ�ȵ�ʵ�鲽�������㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������ȷ�IJ���˳��Ϊ���ڢ٢ۢ�ݢޢߢܣ����Ʋ����г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ�������ʵ�������Ȼ�����ձ����ܽ⣬�ò��������Ͻ�����ٹ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ���������У�500mL����ƿ��
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�500mL����ƿ��
��2��ͼ�г�����ʽ���������������Ʒ����Ϊ����-���룬�پ�ͼ����������20��10��30g������2.6g�������ձ�����Ϊ10+20-2.6=27.4g��
��NaOH��������1.0mol•L-1��NaOH��Һ480mL��Ӧѡ��500ml����ƿ����Ҫ���ʵ�����=1.0mol•L-1��0.5L��40g/mol=20.0g��
�ʴ�Ϊ��27.4��20.0��
��3������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
�ʴ�Ϊ����©��
��4����û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ�
��ת����Һʱ������������������ƿ���棬�������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ�
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ�������Ӱ�죬��Һ�����ʵ���Ũ�Ȳ��䣻
�ܶ���ʱ���ӿ̶��ߣ�������Һ�����ƫС����Һ��Ũ��ƫ�ߣ�
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ�������Һ�����ƫС����Һ��Ũ��ƫ�ߣ�
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫС��
���Բ���������Ũ��ƫ�ߵ��ǣ��ܢݣ���Ӱ����ǣ��ۣ�
�ʴ�Ϊ���ܢݣ��ۣ�
��5���ڵμ�����ˮʱ�����������˿̶��ߣ�Ӧ����ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ��ʴ�Ϊ��ʵ��ʧ�ܣ�ϴ������ƿ�������ƣ�
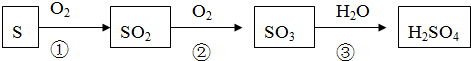
���� ���⿼����һ�����ʵ���Ũ����Һ�����Ƽ�����������ȷ����ԭ�����̼��ɽ����Ŀ�ѶȲ���

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�| A�� | 0.01mol/L�Ĵ�����Һ��c��CO32-��=0.01mol/L | |
| B�� | ��Na2Sϡ��Һ�У�c��H+��=c��OH-��+2c��H2S��+c��HS-�� | |
| C�� | CaCO3������ϡ���ᣬҲ�����ڴ��� | |
| D�� | NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |