��Ŀ����
10�� 25��ʱ��0.1mol•L-1̼������Һ��ͨ��HCl���壬��̼���ӵ�Ũ����pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
25��ʱ��0.1mol•L-1̼������Һ��ͨ��HCl���壬��̼���ӵ�Ũ����pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��pH=7.0ʱ����Һ�к�̼����ֻ��CO32-��HCO3- | |
| B�� | 0.1 mol•L-1Na2CO3��ҺpH=13 | |
| C�� | H2CO3��Ka2=1.0��10-10.25 | |
| D�� | ��100 mL 0.1 mol•L-1̼������Һ�еμ���������ҺpH=4.0�����ɱ�״����CO2����224 mL |
���� A������ͼ���֪��pH=7.0ʱ����Һ����Ҫ����̼��������Ӻ�̼�
B��0.1 mol•L-1Na2CO3��Һ����������Ũ��С��0.1mol/L��
C������Ka2=$\frac{c��C{{O}_{3}}^{2-}��•c��{H}^{+}��}{c��HC{{O}_{3}}^{-}��}$��pH=10.25ʱCO32-��HCO3-��Ũ����Ƚ��м��㣻
D����ҺpH=4.0ʱ����Һ�к�̼������Ҫ��H2CO3��
��� �⣺A����ͼ�����߱仯��֪����pH=7.0ʱ����Һ�к�̼����ֻ��CO32-��H2CO3����A����
B��0.1 mol•L-1Na2CO3��Һ��̼������Ӳ���ˮ�⣬��Һ������������Ũ��С��0.1mol/L��������Һ��pHС��13����B����
C��pH=10.25ʱc��CO32-��=c��HCO3-����Ka2=$\frac{c��C{{O}_{3}}^{2-}��•c��{H}^{+}��}{c��HC{{O}_{3}}^{-}��}$=c��H+��=1.0��10-10.25����C��ȷ��
D����100 mL 0.1 mol•L-1̼������Һ�еμ���������ҺpH=4.0����Һ�к�̼������Ҫ��H2CO3���������ɵĶ�����̼�����һ��С��224 mL����D����
��ѡC��
���� ���⿼�������ӷ�Ӧ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷͼ�����߱仯��ʵ��Ϊ���ؼ���ע������̼���ơ�̼�����Ƶ����ʣ�����������ѧ���ķ�����������ѧ����������

| A�� | ˮ���������H+Ũ��Ϊ1��10-12 mol•L-1����Һ��Na+��Cl-��HCO3- | |
| B�� | ��ʹpH��ֽ������ɫ����Һ��Na+��AlO2-��CO32- | |
| C�� | ���д���Fe3+����Һ��I-��K+��Br- | |
| D�� | pH=2��ˮ��Һ�У�Al3+��NH4+��CH3COO- |
| A�� | H2SO4��H2SO3 | B�� | ��HO��2RO2�ͣ�HO��2RO3 | C�� | HNO3��HNO2 | D�� | H2SiO3��H4SiO4 |
| ���� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ������kJ�� | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
��1���������ʱ������е�������͵���A������ĸ���ţ���
A��H2 B��Cl2C��Br2 D��I2
��2�������⻯�������ȶ�����A������ĸ���ţ���
A��HCl���� ��B��HBr��������C��HI
��3��1molCl2��������H2��ȫ��Ӧʱ�ų� ������ա��ų���������185kJ��
| A�� | �� | B�� | ���� | C�� | �Ȼ�����Һ | D�� | ������ͭ |
 |  |
| A��֤�����ԣ����̼����� | B����ˮ��ɫһ����������ϩ |
 |  |
| C�������������ˮ | D�� ���װ�õ������� |
| A�� | A | B�� | B | C�� | C | D�� | D |

| A�� | ����ʱ��NaHCO3��ˮ�е��ܽ�ȱ�Na2CO3�Ĵ� | |
| B�� | ʯ������Cl2�ķ�Ӧ�������Ʊ�Ư�ۣ�Ư�۵���Ҫ�ɷ���Ca��ClO��2��CaCl2 | |
| C�� | �����¸����Cl2���ø�ƿ���棬����Cl2��������Ӧ | |
| D�� | ͼ����ʾת����Ӧ����������ԭ��Ӧ |
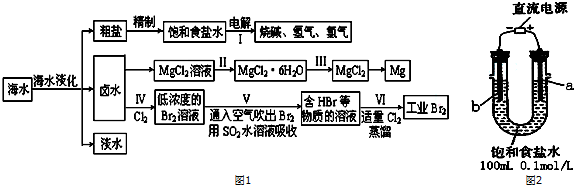
��һ��ij��ѧ�о�С����ͼ2װ��ģ�ⲽ��I���ʳ��ˮ��������ʯī���缫����
��1��a�缫������ʯī��������ʯī������缫��ӦʽΪ2Cl--2e-=Cl2����
��2������������11.2mL����ʱ����״����������Һ��pHΪ12�����Է�Ӧǰ����Һ����ı仯����
������±ˮ���̺��ŷḻ��þ��Դ����MgCl2�ֲ�Ʒ���ᴿ��þ��ұ�����̻ش��������⣺��֪MgCl2�ֲ�Ʒ����Һ�к���Fe2+��Fe3+��Al3+���±��������������������pH��
| ���� | Fe��OH��3 | Fe��OH��2 | Al��OH��3 | Mg��OH��2 |
| ��ʼ����pH | 2.7 | 8.1 | 3.8 | 9.5 |
| ��ȫ����pH | 3.7 | 9.6 | 4.8 | 11.0 |
a��KMnO4 b��H2O2 c��MgO d��NaOH
��4���������MgCl2•H2O���MgCl2�IJ����ǣ���MgCl2•6H2O�ڸ����HCl�����м��ȣ�
��������ȡ��ҵ�壺
��5����������ѻ��Br2����������ֽ�Br2��ԭΪBr-����Ŀ���Ǹ�����Ԫ�أ�
��6��д���������SO2ˮ��Һ����Br2�����ӷ���ʽ��Br2+SO2+2H2O=4H++SO42-+2Br-��