��Ŀ����
13������˵����ȷ���ǣ��������ټ��Է���һ�����м��Լ����Ǽ��Է���һ�����зǼ��Լ�
�ڼ��Է�����һ�������зǼ��Լ����Ǽ��Է����п��ܺ��м��Լ�
��N2O��CO2�ǻ�Ϊ�ȵ����壬���ÿ�����Ӿ�����2���� ����������ԭ�Ӿ�Ϊsp�ӻ�
�ܱ�ͪ�ķе�Ϊ57����ڶ���ķе�Ϊ-0.5�������ڱ�ͪ���Ӽ������
�����ǻ���������ۡ��е�ȶ��ǻ�������ĵ�������йأ�
| A�� | �ۢ� | B�� | �ڢܢ� | C�� | �ڢۢܢ� | D�� | �٢ڢۢܢ� |
���� �ٲ�ͬ�ǽ���Ԫ��֮�����γɼ��Լ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ�������������IJ��غϵķ���Ϊ���Է��ӣ�������������غϵķ���Ϊ�Ǽ��Է��ӣ�
�ڲ�ͬ�ǽ���Ԫ��֮�����γɼ��Լ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ�������������IJ��غϵķ���Ϊ���Է��ӣ�������������غϵķ���Ϊ�Ǽ��Է��ӣ�
�۵ȵ�����Ľṹ���ƣ�
�ܱ�ͪ���Ӽ�û�������
�����ǻ���������ڷ�������������ǻ���������ڷ��Ӽ������
��� �⣺�ټ��Է���һ�����м��Լ����Ǽ��Է��Ӳ�һ�����зǼ��Լ����������̼��ֻ���м��Լ����ṹ�Գ��ǷǼ��Է��ӣ��ʴ���
�ڼ��Է����п��ܺ��зǼ��Լ�������������к���O-O�Ǽ��Լ������ڼ��Է��ӣ��Ǽ��Է����п��ܺ��м��Լ����������̼��ֻ���м��Լ����ṹ�Գ��ǷǼ��Է��ӣ��ʴ���
�۵ȵ�����Ľṹ���ƣ�N2O��CO2�ǻ�Ϊ�ȵ����壬������̼�ĽṹʽΪO=C=O����N2O�ĽṹʽΪ��N=N=O�����ÿ�����Ӿ�����2���� ����������ԭ�Ӿ�Ϊsp�ӻ�������ȷ��
�ܱ�ͪ���Ӽ�û���������ͪ���ӵ���Է����������ڶ��飬���Ա�ͪ�ķе���ڶ��飬������أ��ʴ���
�ݶ��ǻ����������γɷ���֮������������ǻ��������γɷ�����������������ǻ���������ۡ��е�ȶ��ǻ�������ĵͣ�����ȷ��
��ѡA��
���� ���⿼�鹲�ۼ����������������ʵ����ʵ�Ӱ�죬��ȷ�����ҪӰ�����ʵ����������ǽ����Ĺؼ�����Ŀ�ѶȲ���

 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�| A�� | Al | B�� | Na | C�� | Zn | D�� | Fe |
| A�� | 168O��178O��188O����ͬλ�� | |
| B�� | N60��N2��Ϊͬϵ�� | |
| C�� | O2��O3��H2��D2��H218O��H216O����Ϊͬ�������� | |
| D�� | CH2O2��C2H4O2��C3H6O2��C4H8O2��Ϊͬϵ��Ҷ�����������Ҳ��Ϊͬϵ�� |
��1��TiCl4�����������Ѻ��Ѱ�ԭ�ϣ���ҵ����Ҫ��TiO2�Ȼ��ķ�������ȡ��
���������������Ȼ���Ӧ����ȡTiCl4��TiO2��s��+2Cl2��g��?TiCl4��l��+O2��g����H=+151kJ/mol������Ϊ��һ�Ȼ���Ӧ�Ƿ���Է����У����۸�����ʲô����Ӧ�����Է����У���Ϊ�˷�Ӧ�С�H��0����S��0����G=��H-T•��S��0���ʴ˷�Ӧ�����Է����У�
�ڹ�ҵ��ͨ����TiO2��Cl2��Ӧ��ϵ�м���̼���ʣ���һ����������ȡTiCl4���ӻ�ѧƽ��ĽǶȽ��ʹ˷�����˳����ȡTiCl4��ԭ������̼������������Ӧ����С�˲���O2��Ũ�ȣ�ʹTiO2��s��+2Cl2��g��?TiCl4��l��+O2��g��ƽ��������Ӧ�����ƶ���ʹ��Ӧ�ܹ�˳�����У�
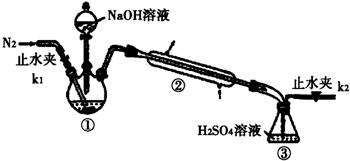
��2��ij��ѧʵ��С����TiO2������ CCl4Ϊԭ����ȡTiCl4��װ��ͼ��ͼ1��

�����й����ʵ����ʣ�
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76.8 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ������������ |
��ʵ�鿪ʼʱ�ȵ�ȼA���ľƾ��ƣ���C����ƿ����Һ�γ���ʱ�ٵ�ȼB���ľƾ��ƣ�����ҪĿ�������ž�ϵͳ��װ�ã��еĿ�����
�ڱ�ʵ��������a��������ļ��ȷ�ʽ��ͻ���ŵ������Ⱦ��ȣ���Ϊ��Ӧ�ṩ�ȶ���CCl4��������
��B��TiO2������Ӧ�Ļ�ѧ����ʽ��TiO2+CCl4$\frac{\underline{\;\;��\;\;}}{\;}$TiCl4+CO2����
��������Cװ���е�TiCl4��Ӧ���õ�ʵ�����Ϊ������������ƣ���
��������Ӧ���������ɵ�����X������������ͨ��Ba�� OH��2��NaOH�Ļ��ϡ��Һ�У����ɳ��������ʵ�����n���� ͨ������X�������V���Ĺ�ϵ��ͼ2��ʾ������ϡ��Һ��Ba�� OH��2��NaOH�����ʵ���֮��Ϊ1��1�� ��a�㵽b�����η����ĵ�һ����Ӧ�����ӷ���ʽ��CO2+2OH-=CO32-+H2O��
| A�� | �ܢݢޢ� | B�� | �ܢݢߢ� | C�� | �ۢܢݢ� | D�� | �ۢܢݢߢ� |