��Ŀ����
13����A+��B-��C2-��D��E��F��G�ֱ��ʾ����10�����ӵ������������ӻ���ӣ����ش��������⣺��1��AԪ����Na��BԪ����F��CԪ����O������Ԫ�ط��ű�ʾ��
��2��D��������Ԫ����ɵ�˫ԭ�ӷ��ӣ������ʽ��HF��
��3��E������10�������������ȶ���ԭ�ӣ���ѧ���ʼ������ã���ԭ�ӽṹʾ��ͼΪ
 ��
����4��F��������Ԫ����ɵ���ԭ�ӷ��ӣ������ʽΪH2O��
��5��һ��G���Ӻ���5��ԭ�ӣ�������Ϊ���飮
���� ����10�����ӵ����У�CH4��NH3��H2O��HF��NH4+��OH-��H3O+��O2-��F-��Na+��Mg2+��Al3+�ȣ����ݸ����ӵ���ɺ����ʽ����⣮
��� �⣺����10�����ӵ����У�CH4��NH3��H2O��HF��NH4+��OH-��H3O+��O2-��F-��Na+��Mg2+��Al3+�ȣ���
��1��A+��ʾ����10�����ӵ�����A+��ʾ����ʧȥһ�����ӣ���ô������������Ϊ11�����Ԫ��Ϊ�ƣ�Na����
B-��ʾ����10�����ӵ�����B-��ʾ����õ�1�����ӣ���ô������������Ϊ9�����Ԫ��Ϊ����F����
C2-��ʾ����10�����ӵ�����C2-��ʾ����õ�2�����ӣ���ô������������Ϊ8�����Ԫ��Ϊ����O����
�ʴ�Ϊ��Na��F��O��
��2��D ��������Ԫ����ɵ�˫ԭ�ӷ��ӣ��Һ���10�����ӣ����������HF���ʴ�Ϊ��HF��
��3��E������10�������������ȶ���ԭ�ӣ���ѧ���ʼ������ã���EΪNe����ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��F��������Ԫ����ɵ���ԭ�ӷ��ӣ�����֪�����ʽH2O���ʴ�Ϊ��H2O��
��5��һ��G���Ӻ���5��ԭ�ӣ������֪�����ʽCH4��������Ϊ���飬�ʴ�Ϊ�����飮
���� ���⿼�鳣��10�������ӵĽṹ�����ʣ���Ŀ�Ѷ��еȣ�ע�ⳣ��10�������Ӻ�18�������ӵĹ�������𣬲����ڿ���ѧ���ķ��������ͶԻ���֪ʶ��Ӧ��������

2NO��g��?N2��g��+O2��g������K1=1��1030��
2H2��g��+O2��g��?2H2O��g����K2=2��1081��
2CO2��g��?2CO��g��+O2��g����K3=4��10-92��
| A�� | ����ʱ��������Ӧ�ķ�Ӧ��Ļ���Ӱٷ��������� | |
| B�� | �����£�ˮ�ֽ����O2����ʱƽ�ⳣ������ֵԼΪ5��10-80 | |
| C�� | �����£�NO��H2O��CO2���ֻ�����ֽ�ų�O2�������ɴ�С��˳��ΪNO��H2O��CO2 | |
| D�� | ����ʱ��������Ӧ�Ļ�ѧ��Ӧ���ʾ����� |
| A�� | C2H4Cl2 | B�� | C5H12 | C�� | C | D�� | C2H5Cl |
 �����������г������л���밴Ҫ������������⣻
�����������г������л���밴Ҫ������������⣻ ��
�� ��A�Ķ�������ͬ���칹����3�֣�
��A�Ķ�������ͬ���칹����3�֣� ���䵥����
���䵥���� ����ṹ��ʽ����д��һ�������¸õ���������������Ӧ�Ļ�ѧ����ʽ
����ṹ��ʽ����д��һ�������¸õ���������������Ӧ�Ļ�ѧ����ʽ ��
��
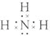 ��
�� ���Ʒ�ȩ��֬����
���Ʒ�ȩ��֬���� ������ɱ�������CH2=CH��CH=CH2���ϳ���ԭ�ϣ���HCHO����������
������ɱ�������CH2=CH��CH=CH2���ϳ���ԭ�ϣ���HCHO����������