��Ŀ����
��ʵ��ȷ��ij��HA�����ᣮ��ͬѧ�ķ����ǣ�
�ף�������0.1mol?L-1��HA��Һ100mL��
����pH��ֽ�������Һ��pH������֤��HA�����ᣮ
�ң�������0.1mol?L-1��HA��Һ100mL������0.1mol?L-1��������Һ100mL
��ȡ��ͬ�����0.1mol?L-1��HA��0.1mol?L-1��������Һ�ֱ�װ����ֻ�Թܣ�ͬʱ����������С��ͬ��п�����۲�������֤��HA�����ᣮ
��1�������������ĵڢٲ��У���Ҫ�õ��Ķ��������� ��
��2�������У�˵��HA������������Dz����Һ��pH 1��ѡ���������������=�������ҷ����У�˵��HA������������� ��
a��װHCl��Һ���Թ��зų�H2�����ʿ�
b��װHA��Һ���Թ��зų�H2�����ʿ�
c����ֻ�Թ��в�����������һ���죮
�ף�������0.1mol?L-1��HA��Һ100mL��
����pH��ֽ�������Һ��pH������֤��HA�����ᣮ
�ң�������0.1mol?L-1��HA��Һ100mL������0.1mol?L-1��������Һ100mL
��ȡ��ͬ�����0.1mol?L-1��HA��0.1mol?L-1��������Һ�ֱ�װ����ֻ�Թܣ�ͬʱ����������С��ͬ��п�����۲�������֤��HA�����ᣮ
��1�������������ĵڢٲ��У���Ҫ�õ��Ķ���������
��2�������У�˵��HA������������Dz����Һ��pH
a��װHCl��Һ���Թ��зų�H2�����ʿ�
b��װHA��Һ���Թ��зų�H2�����ʿ�
c����ֻ�Թ��в�����������һ���죮
���㣺���������ˮ��Һ�еĵ���ƽ��
ר�⣺
��������1������һ�����ʵ���Ũ�ȵ���Һʱ����Ҫ��������100mL����ƿ������Ҫ���������ձ�������������ͷ�ιܣ�
��2�����������ˮ��Һ�ﲿ�ֵ��룬������Һ��������Ũ��С����Ũ�ȣ���Ӧ����������Ũ�ȴ�С�йأ�
��2�����������ˮ��Һ�ﲿ�ֵ��룬������Һ��������Ũ��С����Ũ�ȣ���Ӧ����������Ũ�ȴ�С�йأ�
���
�⣺��1���������Ʒ����ж���Ҫ�õ�����������100mL����ƿ������Ҫ���������ձ�������������ͷ�ιܣ�������Ҫ��ƽ������Ҫ��Ͳ���ʴ�Ϊ��100mL������ƿ��
��2�����������ˮ��Һ�ﲿ�ֵ��룬������Һ��������Ũ��С����Ũ�ȣ����0.1mol/L��HA��Һ��pH��1����˵��HA���ֵ��룬Ϊ������ʣ�
�����ͬ������Ӧʱ����Ӧ����ȡ����������Ũ�ȣ�HCl��������Ũ�ȴ���HA������HCl�ķ�Ӧ���ʽϿ죬��a��ȷ��
�ʴ�Ϊ������a��
��2�����������ˮ��Һ�ﲿ�ֵ��룬������Һ��������Ũ��С����Ũ�ȣ����0.1mol/L��HA��Һ��pH��1����˵��HA���ֵ��룬Ϊ������ʣ�
�����ͬ������Ӧʱ����Ӧ����ȡ����������Ũ�ȣ�HCl��������Ũ�ȴ���HA������HCl�ķ�Ӧ���ʽϿ죬��a��ȷ��
�ʴ�Ϊ������a��
���������⿼��������ʵ��жϣ���ȷǿ������ʵı��������ǵ���̶ȣ��ж�����ķ����У�һ�����ʵ���Ũ������ҺpH��С����������Һ����ԡ�����ͬŨ����ͬԪ����ǿ��Ƚϵ�����ǿ���ȣ�ע�⣨2���з�Ӧ����������Ũ���йأ�������ǿ���أ�Ϊ�״��㣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�����й�����Ԫ���γɵ����ӵ�������ȷ���ǣ�������
| A�����������ӵĵ��Ӳ�ṹ�������⣩һ������һ����ϡ������ԭ����ͬ |
| B�����������ӵĵ��Ӳ���һ��������ԭ�ӵĵ��Ӳ����� |
| C�����������ӵİ뾶һ������ԭ�Ӱ뾶�� |
| D�����������������һ���ǰ˵��ӽṹ |
��һ�ڿ����б�¶����KOH���壬������֪��ˮ7.12%��K2CO32.88%��KOH 90%����������Ʒ1g���뵽46.00mL 1mol/L�����У�������������1.07mol/LKOH��Һǡ���кͣ������кͺ����Һ�ɵù��壨������g��
| A��6.07 | B��2.62 |
| C��4.54 | D��3.43 |
 ����ӦCu��s��+2Ag+��aq���TCu2+��aq��+2Ag��s����Ƴ���ͼ��ʾ��ԭ��أ�����������ȷ���ǣ�������
����ӦCu��s��+2Ag+��aq���TCu2+��aq��+2Ag��s����Ƴ���ͼ��ʾ��ԭ��أ�����������ȷ���ǣ�������| A��KNO3�����е�K+����Cu��NO3��2��Һ |
| B��Ag��������Cu������ |
| C������һ��ʱ���Cu��NO3��2��Һ��c��Cu2+������ |
| D��ȡ�����ź����Ƶ�ָ����Ȼ����ƫת |
H2��I2��һ���������ܷ�����Ӧ��H2��g��+I2��g���T2HI��g����H=-a kJ/mol����˵����ȷ���ǣ�������
��֪��

��֪��

| A��H2��I2��HI�����еĻ�ѧ�����ǷǼ��Թ��ۼ� |
| B���Ͽ�2 mol HI�����еĻ�ѧ����������ԼΪ��c+b+a�� kJ |
| C����ͬ�����£�1 mol H2��g����1mol I2��g��������С��2 mol HI ��g���������� |
| D�����ܱ������м���2 mol H2��g����2 mol I2��g������ַ�Ӧ��ų�������Ϊ2a kJ |
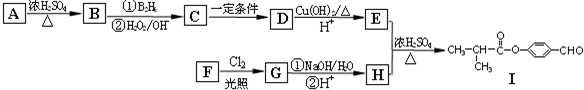
 R-CH2CH2OH��
R-CH2CH2OH��