��Ŀ����
1����ش���1��������ĽṹʽH-O-Cl��
��2����25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH��l��+$\frac{3}{2}$O2��g����CO2��g��+2H2O��l����H=-725.76kJ?mol-1��
��3��SO2����ͨ��BaCl2��HNO3�Ļ����Һ�����ɰ�ɫ��������ɫ���壬�йط�Ӧ�����ӷ���ʽΪ3SO2+2NO3-+3Ba2++2H2O�T3BaSO4��+2NO��+4H+��
��4����Al������������ʯī��������NaHCO3��Һ�����Һ���е�⣬����������R��д����������R�ĵ缫��Ӧʽ��Al+3HCO3--3e-�TAl��OH��3��+3CO2������
���� ��1���������ǹ��ۻ������ԭ�Ӻ���ԭ�ӡ���ԭ�ӷֱ��γ�һ�����ۼ���
��2��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ʱ�ų�����������25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ��32g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ��32=725.76kJ��1mol�״�����Ϊ32�ˣ�������ȫȼ��1mol�״����ɶ�����̼��Һ̬ˮ����725.76KJ������ȼ���ȵĸ���������ɽ��
��3��SO2���н�ǿ��ԭ�ԣ�����ǿ����������HNO3����������ԭ��Ӧ���������ᣬ�����Ȼ����������ᱵ������
��4����ʯī��������AlԪ�ص�����������AlΪ���Ե缫������������Ӧ���缫��ӦʽΪ��Al-3e-=Al3+��Al3+��HCO3-����˫ˮ�⣬����Al��OH��3��
��� �⣺��1���������ǹ��ۻ������ԭ�Ӻ���ԭ�ӡ���ԭ�ӷֱ��γ�һ�����ۼ����ṹʽΪ��H-O-Cl��
�ʴ�Ϊ��H-O-Cl��
��2��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����725.8KJ��ȼ�����Ȼ�ѧ����ʽΪ��CH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H=-725.76 kJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2��g����CO2��g��+2H2O��l����H=-725.76kJ?mol-1��
��3��SO2���н�ǿ��ԭ�ԣ�����ǿ����������HNO3����������ԭ��Ӧ���������ᣬ�����Ȼ����������ᱵ��������Ӧ�����ӷ���ʽΪ3SO2+3Ba2++2H2O+2NO3-=3BaSO4��+2NO��+4H+��
�ʴ�Ϊ��3SO2+2NO3-+3Ba2++2H2O�T3BaSO4��+2NO��+4H+��
��4����ʯī��������AlԪ�ص�����������NaHCO3��Һ�����Һ���е�⣬AlΪ���Ե缫������������Ӧ���缫��ӦʽΪ��Al-3e-=Al3+����������Al3++3HCO3-=Al��OH��3��+3CO2��������Al��OH��3��
�ʴ�Ϊ��Al-3e-=Al3+��Al3++3HCO3-=Al��OH��3��+3CO2����
���� ���⿼�����Ȼ�ѧ����ʽ����д�������ṹʽ����д�����ӷ���ʽ����д����Ŀ�ϼ�ע����������գ�

| ѡ�� | ���� | ���� | ��Ҫ�������� |
| A | �屽 | �� | �������ۺ��壬���� |
| B | �Ȼ��� | �Ȼ�� | ���� |
| C | ���������е�117.7�� | ���ѣ��е�34.5�� | ���� |
| D | �������� | ���� | ���뱥��̼������Һ����Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A | B | C | D |
 |  |  |  |
| ����ͼ��a�����ݼ���N2��ƽ�����������14.5% | ��ʾ��ͨ��ԭ�Ͽ�ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ | ����Ⱥ�������ͨ��N2��H2����t1=��t2ʱ��N2��ת���ʣ�a-b�δ���b-c�� | ͼ��T2��T1 |
| A�� | A | B�� | B | C�� | C | D�� | D |
 �ȱ�����Ҫ���л�����ԭ�ϣ�ʵ�����Ʊ��ȱ��ķ�Ӧԭ����ʵ��װ����ͼ��ʾ������װ������ȥ����
�ȱ�����Ҫ���л�����ԭ�ϣ�ʵ�����Ʊ��ȱ��ķ�Ӧԭ����ʵ��װ����ͼ��ʾ������װ������ȥ���������õ����й��������£�
 +Cl2$\stackrel{FeCl_{3}}{��}$
+Cl2$\stackrel{FeCl_{3}}{��}$ +HCl
+HCl| �е㣨�棩 | �ܶȣ�g•mL-1�� | �ܽ��� | |
| �� | 80.1 | 0.88 | ������ˮ |
| �ȱ� | 132.2 | 1.10 | ������ˮ���������л��ܼ� |
| �Զ��ȱ� | 173.4 | 1.46 | ������ˮ���������л��ܼ� |
| �ڶ��ȱ� | 180.4 | 1.30 | ������ˮ���������л��ܼ� |
�ش��������⣺
��1��Aװ�����Ʊ����������ӷ���ʽΪ2MnO4-+16H++10Cl-=2Mn2+��+5C12��+8H2O��
��2��Bװ����ʢ�ŵ�ҩƷΪCaCl2��P2O5��Cװ���в��õļ��ȷ������Ϊ40��-60���ˮԡ���ȣ�
��3����֪Ũ����ͱ������н�ǿ�Ļӷ��ԣ�������ͼ��ʾװ�ü���Һ����ŵ��Ƿ�ֹҺ��ӷ����ܹ�ƽ��ѹǿ��ʹҺ��˳�����£�
��4��������������Һϴ�ӿ��Գ�ȥ���ʵĻ�ѧʽΪHCl��Cl2��FeCl3�������Ʒϴ�Ӹɾ��ķ���Ϊȡ���һ��ϴ��Һ���Թ��У��������������������ֳ�������֤���Ѿ�ϴ�ɾ���
��5����ʵ�����һ��ȱ�ݣ��Ľ���ʩΪ���������϶��ݳ���HCl��Cl2ͨ��NaOH��Һ�У���ֹ��Ⱦ������
��6����ʵ�������ȱ��IJ���Ϊ44.4%������С�����һλ����
| A�� | �٢ڢܢ� | B�� | �٢ۢݢ� | C�� | �٢ۢܢ� | D�� | �٢ڢۢ� |
| A�� | 1.0LpH=12��CH3COONa��Һ��OH-һ��Ϊ0.01NA | |
| B�� | �����£�20g2H2O���еĵ�����Ϊ10NA | |
| C�� | mgNa2O2��ˮ��Ӧ����1molO2ʱת�Ƶ�����Ϊ2NA | |
| D�� | ��״���£�22.4LN2��CO�Ļ�����к��õ��Ӷ���ĿΪ2NA |
 ��
�� +CO$��_{��}^{AlCl_{3}��HCl}$
+CO$��_{��}^{AlCl_{3}��HCl}$ $��_{OH-����}^{CH_{3}CHO}$B$��_{��H+}^{��C}$
$��_{OH-����}^{CH_{3}CHO}$B$��_{��H+}^{��C}$ $��_{Ũ�����}^{CH_{3}OH}$E
$��_{Ũ�����}^{CH_{3}OH}$E ��
�� B��
B��
 D
D
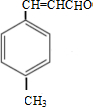
 ��
��
 ��
��