��Ŀ����
10������˵����ȷ���ǣ�������| A�� | ��ѿ�������ǵ�ˮ�������������ǣ��ʶ��߾�Ϊ��ԭ�Զ��� | |
| B�� | �����£���0.4mol/L HA��Һ��0.2mol/LNaOH��Һ�������ϣ���������ı仯����û����Һ��pH=5��������Һ����ˮ�������c��H+��=1��10-5mol/L | |
| C�� | ��̼�²��ϡ�̼������ĭ����ÿ����ĭ����Լ4000��̼ԭ�ӣ�ֱ��Լ6��9nm���ڵ���-183��ʱ����ĭ�������ô��ԣ���̼������ĭ����ʯī��Ϊͬ�������� | |
| D�� | ��֪ Ag2CrO4��KspΪ1.12��10-12���������1��10-4 mol•L-1��AgNO3��Һ��1��10-4 mol•L-1 K2CrO4��Һ��Ϻ����Ag2CrO4�������� |
���� A������ˮ���IJ����������л�ԭ�ԣ�����û�л�ԭ�ԣ�
B�������£���0.4mol/L HA��Һ��0.2mol/LNaOH��Һ�������ϣ����Һ��HA��NaA��Ũ����ȣ������Һ��pH=5����Һ��ʾ���ԣ���Һ������������Ϊˮ����ģ�
C����̼������ĭ������̼������ʯī��Ϊͬ�������壻
D����һ���¶��£�Ksp��һ��������ͨ���Ƚ�Ksp����Һ���й�����Ũ���ݵij˻�--���ӻ�Qc����Դ�С�������ж����ܵ�����ڸ��������³����ܷ����ɻ����ܽ⣬��Qc��Kspʱû�г���������
��� �⣺A������ˮ���IJ����������л�ԭ�ԣ�����û�л�ԭ�ԣ����Բ��ǻ�ԭ�Ͷ��ǣ���A����
B����û����Һ��pH=5��HA��������Һ��ʾ���ԣ���Ӧ��Ļ��Һ��������������ˮ����ģ�����ˮ�����������Ũ��Ϊ��c��H+��=1��10-9mol/L����B����
C����̼������ĭ������̼������ʯī��Ϊͬ�������壬��C��ȷ��
D���������Ϻ�c��Ag+��=5��10-5mol/L��c��CrO42-��=5��10-5mol/L��Qc=c2��Ag+����c��CrO42-��=��5��10-5��2��5��10-5��Ksp��Ag2CrO4��=9.0��10-12����û�г�����������D����
��ѡC��
���� ������Ҫ�����˷��ӽṹ�ı���ģ�ͣ���Һ����Ũ�ȵļ��㣬ͬ����������ж��Լ������ܽ�ƽ����жϵȣ��ѶȲ���ע�����֪ʶ�Ļ��ۣ�

| A�� |  �Ƚ�Cl2��Br2��I2�����������ǿ�� | B�� |  ̽���ռ��ܽ�ʱ����ЧӦ | ||
| C�� |  ����ѧ��ת��Ϊ���� | D�� |  ����һ�����ʵ���Ũ�ȵ���Һ |
| A�� | m��n��w=3��1��2 | |
| B�� | ������¶Ȳ���ʱ������ǰ10s��A������x mol����B��ǰ20s�ڼ���$\frac{2x}{3}$ mol | |
| C�� | ������¶Ȳ���ʱ���淴Ӧ����ѹǿ���½���һ��ʱ���ѹǿ���ֺ㶨���� | |
| D�� | ��λʱ���ڷ�Ӧ��Ũ�ȵļ��ٴ���������Ũ�ȵ����� |
| A�� | ����ˮ�ܼ�Ϳ������л��ܼ�Ϳ�� | |
| B�� | �ÿɽ�������������װ�л��ͺ� | |
| C�� | �÷�Ӧ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CuSO4+SO2��+2H2O �Ʊ�����ͭ | |
| D�� |  +CH2=CH2�� +CH2=CH2�� |
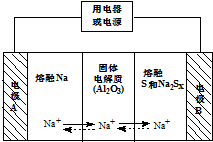 �����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ���ԭ����ͼ��ʾ��Na2SX$?_{�ŵ�}^{���}$2Na+xS ��3��x��5��
�����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ���ԭ����ͼ��ʾ��Na2SX$?_{�ŵ�}^{���}$2Na+xS ��3��x��5��| ���� | Na | S | Al2O3 |
| �۵�/�� | 97.8 | 115 | 2050 |
| �е�/�� | 892 | 444.6 | 2980 |
A��100������ B��100�桫300��C��300�桫350��D��350�桫2050��
��2�����������أ�����˵����ȷ����ACD������ĸ��ţ���
A���ŵ�ʱ���缫AΪ����
B���ŵ�ʱ��Na+���ƶ�����Ϊ��B��A
C���ŵ�ʱ��������ӦʽΪ 2Na-2e-=2Na+
D�����ʱ�缫B�ĵ缫��ӦʽΪSX2--2e-=xS
��3��25��ʱ��������������Ϊ��Դ���200mL 0.2mol/L NaCl��Һ������Һ��pH��Ϊl3ʱ����·��ͨ���ĵ��ӵ����ʵ���Ϊ0.02mol�������ķ�Ӧ���������Ϊ0.92g����������ǰ�����ķ�Ӧ�����������Һ�������仯��

ͼ�е�M�Ƕ����ڽ���Ԫ�أ�M�IJ��ֵ��������±���
| I1 | I2 | I3 | I4 | I5 | |
| ������/kJ•mol-1 | 738 | 1451 | 7733 | 10540 | 13630 |
��1��Ti�Ļ�̬ԭ����Χ�����Ų�ʽΪ3d24s2��
��2��M��Mg����Ԫ�ط��ţ����ý�������Ķѻ�ģ��Ϊ�������ܶѻ�����λ��Ϊ12��
��3������TiO2��һ��Ӧ�ù㷺�Ĵ���������TiO2����һ��ʵ����ͼ2��ʾ��������ķ����в�ȡsp2��ʽ�ӻ���̼ԭ����7�������������в�ȡsp3��ʽ�ӻ���ԭ�Ӷ�Ӧ��Ԫ�صĵ縺���ɴ�С��˳��ΪO��N��C��
��4����һ�ֵ����Ѿ���ľ�����NaCl�������ƣ���ͼ3��ʾ���þ�����N��Ti֮����������Ϊa pm����õ����ѵ��ܶ�Ϊ$\frac{4��62}{{N}_{A}����2a��1{0}^{-10}��^{3}}$g•cm-3��NAΪ�����ӵ�������ֵ��ֻ�м���ʽ�����þ�������Nԭ�Ӿ�������������Nԭ����12����
��5����ѧ��ͨ��Xһ����̽��KCl��MgO��CaO��TiN�ľ�����NaCl�ľ���ṹ���ƣ���KCl��CaO��TiN�������Ӿ����۵��ɸߵ��͵�˳��ΪTiN��CaO��KCl���ж������ǣ����������ĵ����Խ�ߣ�������Խ���۵�Խ�ߣ�
| A | B | C | D | |
| ��Ⱦ | �Ͼ��ȹ������� | úȼ�� | ��Hg2+�Ĺ�ҵ��ˮ | �������� |
| ���� | ������Ϊ���� | ú�м�������ʯ��ʯ | ����Na2S��Һ | ���շ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |

