��Ŀ����
�����ҹ�ũ�巢������ũ������������ʳ����Ѭ�����棬�������ж����嵼�����ж����������¼�����������п���������ҹ�Ŀǰ�����Ѭ��ɱ�������Ҫ�ɷ֣����Ƕ�����ˮ���ᷴӦ�����ж�����PH3��PH3���н�ǿ�Ļ�ԭ�ԣ����ڿ�������ȼ���ҹ���ʳ�������涨����ʳ�������PH3�ƣ�������0.05mg?kg-1����������װ�òⶨ��ʳ�в������ﺬ����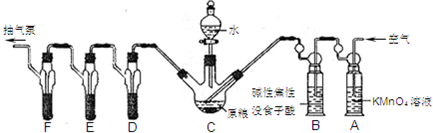
��֪��5PH3+8KMnO4+12H2SO4=5H3PO4+8MnSO4+4K2SO4+12H2O C��ʢ��200gԭ����D��E��F��ʢװ1.00mLŨ��Ϊ1.00��10-3 mol?L-1��KMnO4����Һ��H2SO4�ữ����
��1��д��������ˮ��Ӧ�Ļ�ѧ����ʽ ��
��2���������װ�������Եķ����� ��
��3��ʵ������У��ó����ó�����Ŀ���� ��
��4��A��ʢװKMnO4��Һ��Ϊ��ȥ�����п��ܺ��е� ��B��ʢװ���Խ���ûʳ������Һ�������� ����ȥ��Bװ�ã���ʵ���в�õ�PH3������ ��
��5���ռ�D��E��F��������Һ����ϴ��D��E��F��������Һ��ϴ��Һһ��������ƿ�У���ˮϡ����25mL����Ũ��Ϊ5��10-4 mol?L-1 Na2SO3����Һ�ζ�ʣ���KMnO4��Һ������Na2SO3����Һ11.00mL�����ԭ���������PH3�ƣ��ĺ���Ϊ mg/kg��

��֪��5PH3+8KMnO4+12H2SO4=5H3PO4+8MnSO4+4K2SO4+12H2O C��ʢ��200gԭ����D��E��F��ʢװ1.00mLŨ��Ϊ1.00��10-3 mol?L-1��KMnO4����Һ��H2SO4�ữ����
��1��д��������ˮ��Ӧ�Ļ�ѧ����ʽ
��2���������װ�������Եķ�����
��3��ʵ������У��ó����ó�����Ŀ����
��4��A��ʢװKMnO4��Һ��Ϊ��ȥ�����п��ܺ��е�
��5���ռ�D��E��F��������Һ����ϴ��D��E��F��������Һ��ϴ��Һһ��������ƿ�У���ˮϡ����25mL����Ũ��Ϊ5��10-4 mol?L-1 Na2SO3����Һ�ζ�ʣ���KMnO4��Һ������Na2SO3����Һ11.00mL�����ԭ���������PH3�ƣ��ĺ���Ϊ
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������1����������ˮ���ᷴӦ�����ж�����좣�PH3������ˮ��Ӧ���ˮ�Ľṹ��֪��������ˮ��Ӧ��������������PH3��
��2����������װ���е�ѹǿ�仯�����жϣ�
��3��ȷ�ⶨ좣�PH3���ĺ�����Ҫȫ�����գ�
��4�����������Һ��ǿ�������������ջ�ԭ�����壻���Խ���ûʳ������Һ������ûʳ�����ȺͼӦ���ٺ�������Ӧ����������������������������PH3����������ȼ�գ�
��5���������ĵ������������ʵ�����϶�����ϵ����ʣ�������أ���������PH3��Ҫ�ĸ���������ʵ�������һ������PH3���ʵ������õ�PH3������
��2����������װ���е�ѹǿ�仯�����жϣ�
��3��ȷ�ⶨ좣�PH3���ĺ�����Ҫȫ�����գ�
��4�����������Һ��ǿ�������������ջ�ԭ�����壻���Խ���ûʳ������Һ������ûʳ�����ȺͼӦ���ٺ�������Ӧ����������������������������PH3����������ȼ�գ�
��5���������ĵ������������ʵ�����϶�����ϵ����ʣ�������أ���������PH3��Ҫ�ĸ���������ʵ�������һ������PH3���ʵ������õ�PH3������
���
�⣺��1�����������Ϣ��������ˮ��Ӧ��ˮ������PH3��������������Ӧ�Ļ�ѧ����ʽΪ��AlP+3H2O=Al��OH��3��+PH3����
�ʴ�Ϊ��AlP+3H2O=Al��OH��3��+PH3����
��2����������װ���������������ѹǿ�仯���������ó����ó����۲��װ��������IJ�������������ð����֤����������ã�
�ʴ�Ϊ�������ó������۲��װ�����Ƿ������ݲ�����
��3��ȷ�ⶨPH3�ĺ�������Ҫ�ø��������Һȫ�����գ���������ϴ������Գ������DZ�֤PH3ȫ�������յĴ�ʩ��
�ʴ�Ϊ����֤���ɵ�PH3ȫ��������KMnO4��Һ���գ�
��4������װ��ͼ��װ���е��Լ�ѡ������жϣ����������Һ��ǿ�������������տ����еĻ�ԭ�����壻����ûʳ�����ȺͼӦ���ٺ�������Ӧ����������������������������PH3����������ȼ�գ��õζ������ⶨ��PH3��С�����ƫ�ͣ�
�ʴ�Ϊ����ԭ�����壻��ȥ�����е�O2�� ƫ�ͣ�
��5����ˮϡ����25mL����Ũ��Ϊ5��10-4mol/L Na2SO3����Һ�ζ�ʣ���KMnO4��Һ������Na2SO3����Һ11.00mL�����ݵζ���Ӧ��2KMnO4+5Na2SO3+3H2SO4=2MnSO4+K2SO4+5Na2SO4+3H2O��2KMnO4��5Na2SO3��δ��Ӧ�ĸ���������ʵ���=0.0110L��5��10-4mol/L��
=2.2��10-6mol����PH3��Ӧ�ĸ���������ʵ���=1.00��10-3mol/L��0.0030L-2.2��10-6mol=8.0��10-7mol�����ݷ�Ӧ 5PH3+8KMnO4+12H2SO4=5H3PO4+8MnSO4+4K2SO4+12H2O���õ�������ϵΪ��5PH3��8KMnO4������õ�PH3���ʵ���=8.0��10-7mol��
=5.0��10-7mol����PH3����������=
=0.085g/kg��
�ʴ�Ϊ��0.085��
�ʴ�Ϊ��AlP+3H2O=Al��OH��3��+PH3����
��2����������װ���������������ѹǿ�仯���������ó����ó����۲��װ��������IJ�������������ð����֤����������ã�
�ʴ�Ϊ�������ó������۲��װ�����Ƿ������ݲ�����
��3��ȷ�ⶨPH3�ĺ�������Ҫ�ø��������Һȫ�����գ���������ϴ������Գ������DZ�֤PH3ȫ�������յĴ�ʩ��
�ʴ�Ϊ����֤���ɵ�PH3ȫ��������KMnO4��Һ���գ�
��4������װ��ͼ��װ���е��Լ�ѡ������жϣ����������Һ��ǿ�������������տ����еĻ�ԭ�����壻����ûʳ�����ȺͼӦ���ٺ�������Ӧ����������������������������PH3����������ȼ�գ��õζ������ⶨ��PH3��С�����ƫ�ͣ�
�ʴ�Ϊ����ԭ�����壻��ȥ�����е�O2�� ƫ�ͣ�
��5����ˮϡ����25mL����Ũ��Ϊ5��10-4mol/L Na2SO3����Һ�ζ�ʣ���KMnO4��Һ������Na2SO3����Һ11.00mL�����ݵζ���Ӧ��2KMnO4+5Na2SO3+3H2SO4=2MnSO4+K2SO4+5Na2SO4+3H2O��2KMnO4��5Na2SO3��δ��Ӧ�ĸ���������ʵ���=0.0110L��5��10-4mol/L��
| 2 |
| 5 |
| 5 |
| 8 |
| 5.0��10-7mol��34g/mol |
| 0.2kg |
�ʴ�Ϊ��0.085��
��������������ʳ�в�������Ķ����ⶨΪ���ⱳ��������Ԫ�ػ�����֪ʶ������ԭ��Ӧ�ζ�����ؼ��㣬�ۺϿ�����ˮ�ⷽ��ʽ����д���dz���װ�������Եļ��鷽����ʵ��ԭ��������Ͷ����ⶨ���йؼ�����������ȣ�

��ϰ��ϵ�д�
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
�з�Ӧ4HCl+O2��2Cl2+2H2O����֪2molO2����ԭʱ���ų�a kJ����������֪�Ͽ�1mol
O2��Ҫ����b kJ���������Ͽ�1molCl2��Ҫ����c kJ����������Ͽ�1mol H-O���ȶϿ�1molH-Cl �����������ߣ�������
O2��Ҫ����b kJ���������Ͽ�1molCl2��Ҫ����c kJ����������Ͽ�1mol H-O���ȶϿ�1molH-Cl �����������ߣ�������
A��
| ||
B��
| ||
C��
| ||
D��
|
����˵������ȷ���ǣ�������
| A��12C��13C��14C Ϊ̼Ԫ�ص����ֺ��أ�Ԫ�����ڱ���̼�����ԭ������Ϊ12.01��˵����Ȼ���е�̼��Ҫ��12C�ĺ�����ʽ���ڣ�14CΪ�����Ժ��أ�������ͬλ��ʾ�� |
| B������β����ת��װ�ÿɽ�β���е�NO��CO���к�����ת��ΪN2��CO2����װ���еĴ����ɽ���NO��CO��Ӧ�Ļ�ܣ���������߸÷�Ӧ��ƽ��ת���� |
| C�������ǿ��Ի���ת���ģ�ֲ��Ĺ�����ÿɽ�̫����ת��ɻ�ѧ�ܣ�������ɽ�����ת��ɵ��ܣ�ԭ��غ͵��ؿ�ʵ�ֻ�ѧ�ܺ͵��ܵ��ת�� |
| D����������м����ǻ������Ȼ�����˿���һ�����������۵õ������ᣨPLA�����ø߷��ӻ�������һ�ֿɽ���Ļ������� |





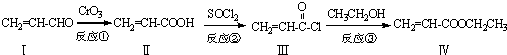
 Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ ����һ����Ҫ���л�ԭ�ϣ��ø����ʿɺϳ��������ʣ�
����һ����Ҫ���л�ԭ�ϣ��ø����ʿɺϳ��������ʣ�
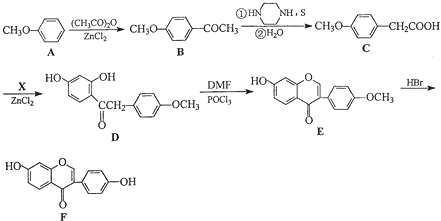
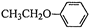 �ͣ�CH3CO��2OΪԭ���Ʊ�ҩ���м���
�ͣ�CH3CO��2OΪԭ���Ʊ�ҩ���м���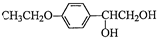 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C=CH2
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C=CH2