��Ŀ����
ʵ�����ô���ʯ��ԭ����ȡ��ȫ����ɱ�����������ƣ�����ʯ����Ҫ���������������������ᴿ����ʯ��ʵ�鲽�裺
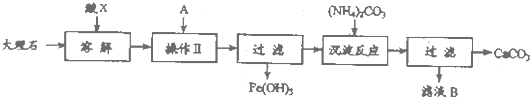
��1����֪��ҺB����Ҫ�ɷ�������泥�����XΪ �������ƣ���ͬ����A����Ϊ ��
��2��������������Һ���Ƿ������ӵ��Լ��� ���ѧʽ�����������۲쵽�������� ��
��3��д������̼�����������Ӧ�����ӷ���ʽ�� ��
��4��CaO2������������д��CaO2��ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
��5��CaO2��һ�㺬CaO��ij̽��С�鰴���й��̲���CaO2���������ȳ�ȡ0.80g��Ʒ��Ȼ����Ʒ����100mL 1.0mol/L�������У��ռ����������ڱ�״���µ����Ϊ112mL�������Ʒ��CaO2����Ϊ ��
��6��Ҫ����100mL 1.0mol/L�����ᣬ��Ҫ12.5mol/L��������Ϊ mL�����Ƹ���Һʱ�����õ���Ͳ���ձ�����ͷ�ι��⣬����Ҫ�IJ�����������Ʒ�� ��

��1����֪��ҺB����Ҫ�ɷ�������泥�����XΪ
��2��������������Һ���Ƿ������ӵ��Լ���
��3��д������̼�����������Ӧ�����ӷ���ʽ��
��4��CaO2������������д��CaO2��ˮ��Ӧ�Ļ�ѧ����ʽ��
��5��CaO2��һ�㺬CaO��ij̽��С�鰴���й��̲���CaO2���������ȳ�ȡ0.80g��Ʒ��Ȼ����Ʒ����100mL 1.0mol/L�������У��ռ����������ڱ�״���µ����Ϊ112mL�������Ʒ��CaO2����Ϊ
��6��Ҫ����100mL 1.0mol/L�����ᣬ��Ҫ12.5mol/L��������Ϊ
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺ʵ�������
��������1����֪��ҺB����Ҫ�ɷ�������泥������ԭ���غ��֪��XΪ���ᣮ��Һ�к��������ӣ�Ҫת��Ϊ������������������Ҫ�����Һ��
��2��������������������������ӻ���ֺ�ɫ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���Fe3+��
��3�����˺����Һ�к��д�����Ca2+������̼��立������ֽⷴӦ����̼��Ƴ�����
��4���������Ƶ����������ڹ������ƣ�
��5����������ˮ��Ӧ�ò������壬ֻ�й���������ˮ��Ӧ������������������������ʵ���������ݷ���ʽCaO2+2H2O=Ca��OH��2+O2����֪���Ĺ������Ƶ����ʵ�����0.005mol������=0.005mol��72g/mol=0.36g�����Ը���Ʒ��CaO2����Ϊ
��100%=45%��
��6���������ʵ����ʵ���������⣬����ʵ�鲽��ȷ��ʵ��������
��2��������������������������ӻ���ֺ�ɫ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���Fe3+��
��3�����˺����Һ�к��д�����Ca2+������̼��立������ֽⷴӦ����̼��Ƴ�����
��4���������Ƶ����������ڹ������ƣ�
��5����������ˮ��Ӧ�ò������壬ֻ�й���������ˮ��Ӧ������������������������ʵ���������ݷ���ʽCaO2+2H2O=Ca��OH��2+O2����֪���Ĺ������Ƶ����ʵ�����0.005mol������=0.005mol��72g/mol=0.36g�����Ը���Ʒ��CaO2����Ϊ
| 0.36g |
| 0.80g |
��6���������ʵ����ʵ���������⣬����ʵ�鲽��ȷ��ʵ��������
���
�⣺��1����֪��ҺB����Ҫ�ɷ�������泥������ԭ���غ��֪��XΪ���ᣮ��Һ�к��������ӣ�Ҫת��Ϊ������������������Ҫ�����Һ��������ҺB������刺�֪��AӦ���ǰ�ˮ���ʴ�Ϊ�������ˮ��
��2��������������������������ӻ���ֺ�ɫ������ʽΪFe3++3SCN-=Fe��SCN��3���ݴ˿��Լ��������ӵĴ��ڣ���ȡ������Һ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���Fe3+����ȡ������Һ�������м������軯����Һ����Һ���ɫ��˵����Һ�к�Fe3+��
�ʴ�Ϊ��KSCN��ȡ������Һ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���Fe3+�� ��ȡ������Һ�������м������軯����Һ����Һ���ɫ��˵����Һ�к�Fe3+��
��3�����˺����Һ�к��д�����Ca2+������̼��立������ֽⷴӦ����̼��Ƴ�������Ӧ�����ӷ���ʽΪCa2++CO32-=CaCO3�����ʴ�Ϊ��Ca2++CO32-=CaCO3����
��4���������Ƶ����������ڹ������ƣ���ˮ��Ӧ�Ļ�ѧ����ʽΪCaO2+2H2O=Ca��OH��2+O2�����ʴ�Ϊ��CaO2+2H2O=Ca��OH��2+O2����
��5����������ˮ��Ӧ�ò������壬ֻ�й���������ˮ��Ӧ�����������������������ʵ���=
=0.005mol������ݷ���ʽCaO2+2H2O=Ca��OH��2+O2����֪���Ĺ������Ƶ����ʵ�����0.005mol������=0.005mol��72g/mol=0.36g�����Ը���Ʒ��CaO2����Ϊ
��100%=45%���ʴ�Ϊ��45%��
��6����ϡ�����������Dz���ģ���Ҫ����100mL 1.0mol/L�����ᣬ��Ҫ12.5mol/L��������Ϊ
=0.008L=8.0ml�����Ƹ���Һʱ�����õ���Ͳ���ձ�����ͷ�ι��⣬����Ҫ�IJ�����������Ʒ��100mL����ƿ�����������ʴ�Ϊ��8.0��100mL����ƿ����������
��2��������������������������ӻ���ֺ�ɫ������ʽΪFe3++3SCN-=Fe��SCN��3���ݴ˿��Լ��������ӵĴ��ڣ���ȡ������Һ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���Fe3+����ȡ������Һ�������м������軯����Һ����Һ���ɫ��˵����Һ�к�Fe3+��
�ʴ�Ϊ��KSCN��ȡ������Һ�������м������軯����Һ����Һ�����ɫ��˵����Һ�в���Fe3+�� ��ȡ������Һ�������м������軯����Һ����Һ���ɫ��˵����Һ�к�Fe3+��
��3�����˺����Һ�к��д�����Ca2+������̼��立������ֽⷴӦ����̼��Ƴ�������Ӧ�����ӷ���ʽΪCa2++CO32-=CaCO3�����ʴ�Ϊ��Ca2++CO32-=CaCO3����
��4���������Ƶ����������ڹ������ƣ���ˮ��Ӧ�Ļ�ѧ����ʽΪCaO2+2H2O=Ca��OH��2+O2�����ʴ�Ϊ��CaO2+2H2O=Ca��OH��2+O2����
��5����������ˮ��Ӧ�ò������壬ֻ�й���������ˮ��Ӧ�����������������������ʵ���=
| 0.112L |
| 22.4L/mol |
| 0.36g |
| 0.80g |
��6����ϡ�����������Dz���ģ���Ҫ����100mL 1.0mol/L�����ᣬ��Ҫ12.5mol/L��������Ϊ
| 1.0mol/L��0.1L |
| 12.5mol/L |
���������⿼���˴���ʯ���ᴿ���漰ʵ�鷽����ʵ����������⣬����ʱע�������Ŀ�еĹؼ���Ϣ�������Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
���з�����ᴿ�ķ�����ȷ���ǣ�������
| A�������dz�ȥˮ�����ʵõ�������ˮ����÷��� |
| B���ù��˵ķ�����ȥNaCl��Һ�к��е��������۽��� |
| C�����ܽ⡢���˵ķ����ᴿ��������BaSO4��BaCO3 |
| D���ü��ȡ������ķ������Գ�ȥ�����е�CaCl2��MgCl2������ |
�����йػ�ܵ�˵����ȷ���ǣ�������
| A��Ag+��Cl-��ˮ��Һ�л�ϣ��䷴Ӧ�Ļ�ܽӽ����� |
| B����ܵĴ�С�Ի�ѧ��Ӧǰ��������仯�ܲ���Ӱ�� |
| C���Ӵ����ܽ�������Ӧ�Ļ�ܣ����������淴Ӧ�Ļ�� |
| D���¶Ƚ��ͣ���Ӧ���ʼ�������ԭ���ǽ����˷�Ӧ�Ļ�� |
 ̼���仯����Ӧ�ù㷺��
̼���仯����Ӧ�ù㷺��
 A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ��
A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ�� ��ͼ���³İ�ֽ�IJ���Ƭ�������������KMnO4����������Χ�ֱ�μ�һ��KBr��Һ��Ʒ����Һ�����з�̪�ij���ʯ��ˮ��FeC12��Һ��Ȼ����KMnO4�����ϵμ�������Ũ���ᣬѸ�ٸǺñ�������ʾ��ʵ���������õ�������������������ԭ����ȡ��
��ͼ���³İ�ֽ�IJ���Ƭ�������������KMnO4����������Χ�ֱ�μ�һ��KBr��Һ��Ʒ����Һ�����з�̪�ij���ʯ��ˮ��FeC12��Һ��Ȼ����KMnO4�����ϵμ�������Ũ���ᣬѸ�ٸǺñ�������ʾ��ʵ���������õ�������������������ԭ����ȡ��