��Ŀ����
7��[Cu��NH3��4]SO4•H2O��һ����Ҫ��Ⱦ�ϼ��ϳ�ũҩ�м��壬���ȿɷֽ⣬Ħ������Ϊ246g•mol-1��ij��ѧ����С�����������ʵ�飨���ּг�װ���ԣ���֤���IJ��ַֽ�����ش����⣺
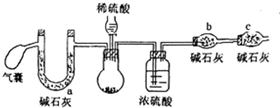
��1������װ�ã����װ�������ԣ��ڸ�װ���м�����Ӧ��ҩƷ���Լ���
��2����K2��K4���պ�K1��K3������һ��ʱ���۲쵽Ʒ����Һ��ɫ��д��NaOH��Һ�з�����Ӧ�����ӷ���ʽSO2+2OH-=SO32-+H2O��
��3����K1��K3���պ�K2��K4����������һ��ʱ���۲쵽ʪ���ɫʯ����ֽ������֤���ֽ�����к���NH3���ѧʽ����
��4��CCl4�������Ƿ�ֹ������
��5�����ȹ����У�������Cu��N2��H2O��д��[Cu��NH3��4]SO4•H2�ֽ�Ļ�ѧ����ʽ3[Cu��NH3��4]SO4•H2O $\frac{\underline{\;\;��\;\;}}{\;}$3Cu+8NH3��+2N2��+3SO2��+9H2O��
��6����ʵ���г�ȡag[Cu��NH3��4]SO4•H2O�ռ���bmLN2���ѻ���ɱ�״������[Cu��NH3��4]SO4•H2O�ķֽ��ʱ���ʽΪ$\frac{3��246��b��1{0}^{-3}}{2��22.4��a}$��100%��
��7�������ܶ���ʱ��ע��������У���������ȴ�����£�������װ������Һ����ƽ���������밼Һ����ʹ���ƽ����ijͬѧ��ͼ2��ʾ�������������ķֽ���ƫ�ͣ��ƫ�ߡ���ƫ�͡�����Ӱ�족����
���� ��1�����������������ɣ���װ��ͼ��֪����������������IJⶨ������ҩƷǰ��Ҫ����װ�������ԣ�
��2���۲쵽Ʒ����Һ��ɫ��˵���ֽ����ɶ�����������������Һ���ն������������������ƣ����ں�����������ⶨ��
��3���۲쵽ʪ���ɫʯ����ֽ������˵���ֽ����ɰ�����
��4����ˮ�������ɰ������Ա��ں�����������IJⶨ����������������ˮ��ֱ����ˮ���ջᷢ����������5�����ȹ����У�������Cu��N2��H2O��������������֪�а����������������ɣ�
��6�����ݵ������������Ϸ���ʽ����ֽ��[Cu��NH3��4]SO4•H2O������������������ֽ��ʣ�
��7����������Ҫ�����밼Һ����ʹ���ƽ��
��ijͬѧ��ͼ2��ʾ�����������ѹǿ���ڴ���ѹ�����屻ѹ�����ⶨ��������ƫС��
��� �⣺��1�����������������ɣ���װ��ͼ��֪����������������IJⶨ������ҩƷǰ��Ҫ����װ�������ԣ�
�ʴ�Ϊ�����װ�������ԣ�
��2���۲쵽Ʒ����Һ��ɫ��˵���ֽ����ɶ�����������������Һ���ն������������������ƣ����ں�����������ⶨ����Ӧ���ӷ���ʽΪ��SO2+2OH-=SO32-+H2O��
�ʴ�Ϊ��SO2+2OH-=SO32-+H2O��
��3���۲쵽ʪ���ɫʯ����ֽ������˵���ֽ����ɰ�����
�ʴ�Ϊ��NH3��
��4����ˮ�������ɰ������Ա��ں�����������IJⶨ����������������ˮ��ֱ����ˮ���ջᷢ�������������������Ȼ�̼�У������������Ȼ�̼���գ����Է�ֹ������
�ʴ�Ϊ����ֹ������
��5�����ȹ����У�������Cu��N2��H2O��������������֪�а����������������ɣ�[Cu��NH3��4]SO4•H2�ֽ�Ļ�ѧ����ʽΪ��3[Cu��NH3��4]SO4•H2O $\frac{\underline{\;\;��\;\;}}{\;}$3Cu+8NH3��+2N2��+3SO2��+9H2O��
�ʴ�Ϊ��3[Cu��NH3��4]SO4•H2O $\frac{\underline{\;\;��\;\;}}{\;}$3Cu+8NH3��+2N2��+3SO2��+9H2O��
��6����ֽ��[Cu��NH3��4]SO4•H2O������Ϊm����
3[Cu��NH3��4]SO4•H2O $\frac{\underline{\;\;��\;\;}}{\;}$3Cu+8NH3��+2N2��+3SO2��+9H2O
3��246g 2��22.4L
m b��10-3L
����m=$\frac{3��246g��b��1{0}^{-3}L}{2��22.4L}$=$\frac{3��246��b��1{0}^{-3}}{2��22.4}$g
[Cu��NH3��4]SO4•H2O�ķֽ��ʱ���ʽΪ��$\frac{3��246��b��1{0}^{-3}}{2��22.4}$g��ag����100%=$\frac{3��246��b��1{0}^{-3}}{2��22.4��a}$��100%��
�ʴ�Ϊ��$\frac{3��246��b��1{0}^{-3}}{2��22.4��a}$��100%��
��7����������Ҫ�����밼Һ����ʹ���ƽ��
��ijͬѧ��ͼ2��ʾ�����������ѹǿ���ڴ���ѹ�����屻ѹ�����ⶨ��������ƫС������ֽ��[Cu��NH3��4]SO4•H2O������ƫС����[Cu��NH3��4]SO4•H2O�ķֽ���ƫ�ͣ�
�ʴ�Ϊ�������밼Һ����ʹ���ƽ��ƫ�ͣ�
���� ���⿼����֤��ʵ�鷽�����ֽ����йؼ��㡢Ԫ�ػ��������ʡ���ԭ����װ�õķ������ۡ�����������ѧʵ����������ȣ��ǶԻ�ѧʵ����ۺϿ��飬�ؼ��������װ�����ã��ϺõĿ���ѧ��ʵ��������������������������

| A�� | �����ն� | B�� | �⻯ѧ���� | C�� | ���� | D�� | ��ɽ���� |
| A�� | 8.4g NaHCO3�����к��е�������������Ϊ0.2 NA | |
| B�� | ��״���£�22.4L���к��еķ�����ΪNA | |
| C�� | ͨ��״���£�NA ���������ռ�е����Ϊ22.4L | |
| D�� | 1L���ʵ���Ũ��Ϊ1mol/L��Na2CO3��Һ�У�����CO32-����ΪNA |
��l�� C��s��+$\frac{1}{2}$O2��g��=CO��g����H=��H1
��2��2H2��g��+O2��g��=2H2O��g����H=��H2
�ɴ˿�֪ C��s��+H2O��g���TCO��g��+H2��g����H3�����H3���ڣ�������
| A�� | ��H1-��H2 | B�� | ��H1-$\frac{1}{2}$��H2 | C�� | 2��H1-��H2 | D�� | $\frac{1}{2}$��H2-��H1 |
�����ô�������β���е�NO��COת���CO2��N2����ѧ����ʽ���£�
2NO��g��+2CO��g��$\stackrel{����}{?}$ 2CO2��g��+N2��g����H=-748kJ/mol
Ϊ�˲ⶨij���������µķ�Ӧ���ʣ���һ���¶��£���ij�����ܱ������г�������ʵ�����NO��CO����������Ӧ�������崫������ò�ͬʱ��NOŨ�������
| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | �� |
| c��NO��/mol•L-1 | 1.00��10-3 | 4.00��10-4 | 1.70��10-4 | 1.00��10-4 | 1.00��10-4 | �� |
��2���ﵽƽ��ʱ�����д�ʩ�����NOת���ʵ���BD��������ĸ��ţ�
A��ѡ�ø���Ч�Ĵ���B�����ͷ�Ӧ��ϵ���¶�
C���������ʹ������ѹǿ����D������COʹ������ѹǿ����
��3����֪N2��g��+O2��g��=2NO��g����H=+180kJ/mol����CO��ȼ����Ϊ284kJ/mol���H=-284kJ/mol��
����Ҳ�����ڴ���NO��
��4��O3����NO���ˮϴ�ɲ���HNO3��O2��ÿ����1mol��HNO3ת��3mol���ӣ�
��5��O3���ɵ��ϡ�����Ƶã�ԭ����ͼ��ͼ������ΪB���A����B���������������Ե缫���ĵ缫��ӦʽΪ3H2O-6e-=O3+6H+��

 �������ƿ�������ɴ��Ĺ����������䴿��Ҫ��ܸߣ�ijС��ͬѧΪ�˲ⶨ�������ƵĴ��ȣ�����Ϊ̼���ƣ�����������·�����
�������ƿ�������ɴ��Ĺ����������䴿��Ҫ��ܸߣ�ijС��ͬѧΪ�˲ⶨ�������ƵĴ��ȣ�����Ϊ̼���ƣ�����������·���������һ��ȡm1g��Ʒ������ ������CaCl2��Һ����ַ�Ӧ����ˡ�ϴ�ӡ�������CaCO3��������Ϊm2g��
��������ȡm1g��Ʒ��������װ�ò�����ɶ�����̼����Ϊm3g��
��������ȡm1g��Ʒ����ˮ����ܽⲢ�������ٲ������壬��cmol/L���������Һ�ζ�������Һ��������ָʾ�������յ�ʱ������������ΪVmL��
�ش��������⣺
��1������һ�У�����ȷ������õĹ������ƵĴ��ȱ�ʵ�ʵ�ƫ�ͣ���ԭ���������ܵ�Ca��OH��2����ʹm2��ֵƫ��
��2��������������Ҫʹ�����Σ��ڶ���ʹ�õ�Ŀ�����ų�װ�������ɵ�CO2��ȫ����ʯ�����գ�C����ܵ������Ƿ�ֹ������ˮ�Ͷ�����̼����b�У�����ϡ�������ϡ���ᣬ��ⶨ���ƫ�ͣ���ߡ������͡���Ӱ�족��
��3���������У��ζ��յ����������Һ�ɻ�ɫ��ɳ�ɫ���Ұ�����ڲ���ƣ���ù������ƵĴ���Ϊ$\frac{39��0.053Vc-{m}_{1}��}{14{m}_{1}}$��100%�����ú�m1��c��V��ʽ�ӱ�ʾ��
��4��ijС��ͬѧ�����������ˮ��Ӧ����Һ�еμӷ�̪��������Һ�ȱ�����ɫ����Ե�����Һ��ɫ��ԭ��������ּ��裺
����һ��������������Һ��Ũ�ȹ����ʹ��Һ��ɫ
��������������˹��������ʹ��Һ��ɫ
��
ʵ����֤��������Ũ�ȷֱ�Ϊ5mol•L-1��2mol•L-1��1mol•L-1��0.01mol•L-1������������Һ�еμӷ�̪��Һ���۲쵽��Һ������ɫ��ʱ�����£�
| ��������Ũ�ȣ�mol•L-1�� | 5 | 2 | 1 | 0.01 |
| ������ɫ��ʱ�䣨s�� | 8 | 94 | 450 | ��ʱ�䲻��ɫ |
���ʵ����֤�����ȡ���ݵ����ķ�ӦҺ���Թ��У�������һ֧�Թܼ��������������̲��ȣ��μ��η�̪����Һ����Ҳ���ɫ����һ֧�Թ���ֱ�Ӽ��뼸�η�̪����Һ��������ɫ��˵�������������
| A�� | C3H8 | B�� | C4H10 | C�� | C5H12 | D�� | C6H14 |
| A�� | �� | B�� | ʳ�� | C�� | ʯ�� | D�� | ���� |

 ��A�ĵ���ʽΪ
��A�ĵ���ʽΪ ��
�� +CH3CH2OH$��_{��}^{Ũ����}$
+CH3CH2OH$��_{��}^{Ũ����}$ +H2O��
+H2O�� $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ ��
�� ����һ�֣���д��һ�ּ��ɣ�
����һ�֣���д��һ�ּ��ɣ�