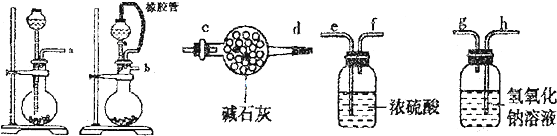
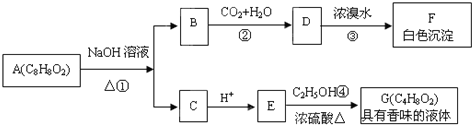
��Ŀ����
���и���������ָ����Һ�У�һ���ܴ���������ǣ�������
��c��H+��=10-12 mol?L-1����Һ�У�I-��NO3-��SO32-��K+
��������Һ�У�Fe3+��NO3-��Na+��SO42-
����ˮ�������c��H+��=10-12mol/L����Һ��Ba2+��Na+��NO3-��Cl-
��ʹ���ȱ��ɫ����Һ�У�CO32-��Na+��AlO2-��SO42-��
��c��H+��=10-12 mol?L-1����Һ�У�I-��NO3-��SO32-��K+
��������Һ�У�Fe3+��NO3-��Na+��SO42-
����ˮ�������c��H+��=10-12mol/L����Һ��Ba2+��Na+��NO3-��Cl-
��ʹ���ȱ��ɫ����Һ�У�CO32-��Na+��AlO2-��SO42-��
| A���ڢ� | B���٢� | C���٢� | D���ۢ� |
���㣺���ӹ�������
ר�⣺
��������c��H+��=10-12 mol?L-1����Һ�ʼ��ԣ�
��Fe3+ˮ������ԣ�
����ˮ�������c��H+��=10-12mol/L����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�
�ܼ��ȵı�ɫ��ΧΪ3.1��4.4��ʹ���ȱ��ɫ����Һ�����ܳ����ԡ����Ի���ԣ�
��Fe3+ˮ������ԣ�
����ˮ�������c��H+��=10-12mol/L����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�
�ܼ��ȵı�ɫ��ΧΪ3.1��4.4��ʹ���ȱ��ɫ����Һ�����ܳ����ԡ����Ի���ԣ�
���
�⣺��c��H+��=10-12 mol?L-1����Һ�ʼ��ԣ���������������֮�䲻�����κη�Ӧ���ɴ������棬����ȷ��
��Fe3+ˮ������ԣ������ܴ��������Ի����У��ʴ���
����ˮ�������c��H+��=10-12mol/L����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�����֮�䲻�����κη�Ӧ���ɴ������棬����ȷ��
�ܼ��ȵı�ɫ��ΧΪ3.1��4.4��ʹ���ȱ��ɫ����Һ�����ܳ����ԡ����Ի���ԣ����������£�CO32-��AlO2-�����ȶ����ڣ��ʴ���
��ѡC��
��Fe3+ˮ������ԣ������ܴ��������Ի����У��ʴ���
����ˮ�������c��H+��=10-12mol/L����Һ��ˮ�ĵ����ܵ����ƣ���Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�����֮�䲻�����κη�Ӧ���ɴ������棬����ȷ��
�ܼ��ȵı�ɫ��ΧΪ3.1��4.4��ʹ���ȱ��ɫ����Һ�����ܳ����ԡ����Ի���ԣ����������£�CO32-��AlO2-�����ȶ����ڣ��ʴ���
��ѡC��
���������⿼�����ӵĹ��棬Ϊ��Ƶ���㣬��ȷ��Ϣ���ھ��Ӧ���ǽ����Ĺؼ���ע�Ȿ���з�����������ԭ��Ӧ���ɽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
����������ˮ��Һ���ܴ���������ǣ�������
| A��Ca2+��K+��Cl-��CO32- |
| B��Ba2+��Cl-��K+��SO42- |
| C��Fe3+��Cl-��K+��NO3- |
| D��Ag+��NO3-��K+��Cl- |
����˵����ȷ���ǣ�������
| A��ֻ����һ��Ԫ�ص�����һ���ǵ��� |
| B����������Һ�ı������������Ƿ��ж�������� |
| C��һ���¶ȡ�ѹǿ�£���������������ʵ����Ķ��پ��� |
| D����ʢ�з�ˮ���ձ��еμ�FeCl3��Һ����ʱ����У��Ʊ�Fe��OH��3���� |
������Һ�и�����Ũ�ȹ�ϵ��ȷ���ǣ�������
| A���������м�����������õ������Ի����Һ��c��Na+����c��CH3COO-����c��H+����c��OH-�� |
| B��ij��Ԫ�������ʽ��NaHA��Һ�У�c��H+��+c��Na+��=c��OH-��+c��HA-��+c��A2-�� |
| C��1.0 mol?L -1Na2CO3��Һ��c��OH-��=2c��HCO3-��+c��H+��+c��H2CO3�� |
| D�����ʵ���Ũ����ȵģ�NH4��2SO4��NH4HSO4��NH4Cl��Һ��c��NH4+������NH4��2SO4��NH4HSO4��NH4Cl |