��Ŀ����
ҩ���л���AΪһ����ɫҺ�壬��A�����ɷ�������һϵ�з�Ӧ
��ش�
��1��������D�ṹ��ʽ�� ��������E�еĹ��������ƣ� ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ�� ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ�� ��
��4���л���A��ͬ���������࣬�����з�����ͷ��������Ը�дһ�ֽṹ��ʽ�� ��
��5��E��һ��ͬ���칹��H����֪H���Ժͽ����Ʒ�Ӧ�ų�����������һ�������¿ɷ���������Ӧ����д��H��������Ӧ����ʽ�� ��

��ش�
��1��������D�ṹ��ʽ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ��
��4���л���A��ͬ���������࣬�����з�����ͷ��������Ը�дһ�ֽṹ��ʽ��
��5��E��һ��ͬ���칹��H����֪H���Ժͽ����Ʒ�Ӧ�ų�����������һ�������¿ɷ���������Ӧ����д��H��������Ӧ����ʽ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
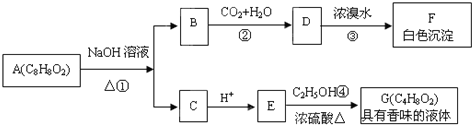
������B�ܺͶ�����̼��ˮ��Ӧ����D��D�ܺ���ˮ����ȡ����Ӧ���ɰ�ɫ������˵��D�к��з��ǻ�������A�к��б�����A�IJ����Ͷ�=
=5����A�л�����һ�������ͼ���A�ܺ��������Ƶ�ˮ��Һ����ˮ�ⷴӦ����B��C����A�к������������A�ķ���ʽ��֪��Ӧ����������γɵ�������C�ữ������E��E�����ᣬE���Ҵ�����������Ӧ����G��G�ķ���ʽΪC4H8O2����E�Ľṹ��ʽΪCH3COOH��CΪCH3COONa��G�Ľṹ��ʽΪCH3COOCH2CH3�����A �ķ���ʽ��֪��AΪ ����BΪ
����BΪ ��DΪ
��DΪ ��FΪ�Ľṹ��ʽΪ
��FΪ�Ľṹ��ʽΪ ���ݴ˽��
���ݴ˽��
| 8��2+2-8 |
| 2 |
 ����BΪ
����BΪ ��DΪ
��DΪ ��FΪ�Ľṹ��ʽΪ
��FΪ�Ľṹ��ʽΪ ���ݴ˽��
���ݴ˽�����
�⣺B�ܺͶ�����̼��ˮ��Ӧ����D��D�ܺ���ˮ����ȡ����Ӧ���ɰ�ɫ������˵��D�к��з��ǻ�������A�к��б�����A�IJ����Ͷ�=
=5����A�л�����һ�������ͼ���A�ܺ��������Ƶ�ˮ��Һ����ˮ�ⷴӦ����B��C����A�к������������A�ķ���ʽ��֪��Ӧ����������γɵ�������C�ữ������E��E�����ᣬE���Ҵ�����������Ӧ����G��G�ķ���ʽΪC4H8O2����E�Ľṹ��ʽΪCH3COOH��CΪCH3COONa��G�Ľṹ��ʽΪCH3COOCH2CH3�����A �ķ���ʽ��֪��AΪ ����BΪ
����BΪ ��DΪ
��DΪ ��FΪ�Ľṹ��ʽΪ
��FΪ�Ľṹ��ʽΪ ��
��
��1��������������֪��������D�ṹ��ʽΪ�� ��������EΪCH3COOH�����еĹ���������Ϊ���Ȼ����ʴ�Ϊ��
��������EΪCH3COOH�����еĹ���������Ϊ���Ȼ����ʴ�Ϊ�� ���Ȼ���
���Ȼ���
��2����Ӧ�ٵĻ�ѧ����ʽΪ�� +2NaOH
+2NaOH
 ++CH3COONa+H2O��
++CH3COONa+H2O��
�ʴ�Ϊ�� +2NaOH
+2NaOH
 +CH3COONa+H2O��
+CH3COONa+H2O��
��3����Ӧ�����������Ҵ�����������Ӧ����������������Ӧ��ѧ����ʽΪ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��
��4���л���A�� ����ͬ���������࣬�����з�����ͷ�����������һ�ֽṹ��ʽ�ֱ�Ϊ��
����ͬ���������࣬�����з�����ͷ�����������һ�ֽṹ��ʽ�ֱ�Ϊ�� ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��5��E��CH3COOH����һ��ͬ���칹��H�����Ժͽ����Ʒ�Ӧ�ų�����������һ�������¿ɷ���������Ӧ������-OH��-CHO��H�Ľṹ��ʽΪHOCH2CHO��H����������Ӧ����ʽΪ��HOCH2CHO+2Ag��NH3��2OH
HOCH2COONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��HOCH2CHO+2Ag��NH3��2OH
HOCH2COONH4+2Ag��+3NH3+H2O��
| 8��2+2-8 |
| 2 |
 ����BΪ
����BΪ ��DΪ
��DΪ ��FΪ�Ľṹ��ʽΪ
��FΪ�Ľṹ��ʽΪ ��
����1��������������֪��������D�ṹ��ʽΪ��
 ��������EΪCH3COOH�����еĹ���������Ϊ���Ȼ����ʴ�Ϊ��
��������EΪCH3COOH�����еĹ���������Ϊ���Ȼ����ʴ�Ϊ�� ���Ȼ���
���Ȼ�����2����Ӧ�ٵĻ�ѧ����ʽΪ��
 +2NaOH
+2NaOH| ˮ |
| �� |
 ++CH3COONa+H2O��
++CH3COONa+H2O���ʴ�Ϊ��
 +2NaOH
+2NaOH| ˮ |
| �� |
 +CH3COONa+H2O��
+CH3COONa+H2O����3����Ӧ�����������Ҵ�����������Ӧ����������������Ӧ��ѧ����ʽΪ��CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
��4�����A��
 ����ͬ���������࣬�����з�����ͷ�����������һ�ֽṹ��ʽ�ֱ�Ϊ��
����ͬ���������࣬�����з�����ͷ�����������һ�ֽṹ��ʽ�ֱ�Ϊ�� ��
�� ��
���ʴ�Ϊ��
 ��
�� ��
����5��E��CH3COOH����һ��ͬ���칹��H�����Ժͽ����Ʒ�Ӧ�ų�����������һ�������¿ɷ���������Ӧ������-OH��-CHO��H�Ľṹ��ʽΪHOCH2CHO��H����������Ӧ����ʽΪ��HOCH2CHO+2Ag��NH3��2OH
| �� |
�ʴ�Ϊ��HOCH2CHO+2Ag��NH3��2OH
| �� |
���������⿼���л����ƶϣ�ע����ݷ����ķ�Ӧ�жϺ��еĹ����ţ�����л������ʽ�ƶϣ���Ҫѧ���������չ����ŵ�������ת�����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 25��ʱ��ȡŨ�Ⱦ�Ϊ0.1mol?L-1�Ĵ�����Һ�Ͱ�ˮ��Һ��20mL���ֱ���Ũ�Ⱦ�Ϊ0.1mol?L-1NaOH��Һ����������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ����ͼ��ʾ������˵������ȷ���ǣ�������
25��ʱ��ȡŨ�Ⱦ�Ϊ0.1mol?L-1�Ĵ�����Һ�Ͱ�ˮ��Һ��20mL���ֱ���Ũ�Ⱦ�Ϊ0.1mol?L-1NaOH��Һ����������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ����ͼ��ʾ������˵������ȷ���ǣ�������| A�����ߢμ���Һ��20 mLʱ��Һ��pH=5��c��H+��-c����NH3?H2O��=c��OH-��=1��10-9 mol?L-1 |
| B�����ߢμ���Һ��20 mLʱ��c��Cl-����c��NH4+����c��H+����c��OH-�� |
| C�����ߢμ���Һ��10 mL��20 mL֮����ڣ�c��NH4+��=c��Cl-����c��OH-��=c��H+�� |
| D�����ߢμ���Һ��10 mLʱ��c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��] |


 A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1�� Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ�EΪ��������δ�ɶԵ���������Ԫ�أ���ش��������⣺
A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1�� Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ�EΪ��������δ�ɶԵ���������Ԫ�أ���ش��������⣺ ��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E�����Ϸ�Ӧ��B�Ľ�һ����Ӧ��ͼ��ʾ��
��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E�����Ϸ�Ӧ��B�Ľ�һ����Ӧ��ͼ��ʾ��
 X��Y��Z��M��N��K���ɶ�����Ԫ�ع��ɵ���������X��Y��Z�������ӣ�M��N�����Է��ӣ�K�������ӣ����Ǿ������нṹ�ص�����ʣ�
X��Y��Z��M��N��K���ɶ�����Ԫ�ع��ɵ���������X��Y��Z�������ӣ�M��N�����Է��ӣ�K�������ӣ����Ǿ������нṹ�ص�����ʣ�