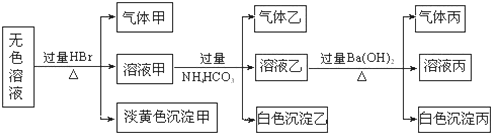
��Ŀ����
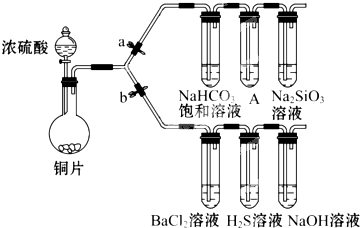 ����Ҫ��������и�С��ʵ��Ŀ�ģ���a��b Ϊ���ɼУ����ȼ��̶�װ������ȥ��
����Ҫ��������и�С��ʵ��Ŀ�ģ���a��b Ϊ���ɼУ����ȼ��̶�װ������ȥ����1����֤̼����ǽ����Ե����ǿ��������֪���ԣ������̼�ᣩ
������������
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
װ��A�е��Լ���
����˵��̼�ķǽ����Աȹ�ǿ��ʵ�������ǣ�
��2����֤ SO2�������ԡ���ԭ�Ժ������������ͨ�ԣ�
�ٴ�b���ر�a������֤SO2���������ԵĻ�ѧ����ʽ�ǣ�
����������SO2ͨ��NaOH��Һ�У��仯ѧ����ʽ�ǣ�
��BaCl2��Һ������������ֳ����ݣ��քe�μ�������Һ���������ij����Ļ�ѧʽ�����±���Ӧλ�ã�
| �μӵ���Һ | �ȡ�ˮ | ����ˮ |
| �����Ļ�ѧʽ |
���㣺����ʵ�鷽�������
ר�⣺ʵ�������
��������1������֤̼����ǽ����Ե����ǿ������ͨ��̼��Ϳ����Թ����η�Ӧ�����������ʵ�ֵģ�����Ϊ�˱�֤ʵ��Ч����ʵ���˳�����У�����װ�õ������ԣ�
��ͭ��Ũ���ᷴӦ��ͭ��������+2�۵�ͭ���ӣ����ᱻ��ԭ��+4�۵Ķ��������������ǿ�����ԣ���������������
�۵�A��KMnO4��Һû����ȫ��ɫ��˵�����������Ѿ���ȫ������ʢ��Na2SiO3��Һ���Թ��г��ְ�ɫ������˵��̼�ķǽ����Աȹ�ǿ��
��2���ٶ�����������Ԫ�صĻ��ϼ���+4�ۣ���������-2�۵���ԭ��
����������SO2ͨ��NaOH��Һ�У�����NaHSO3��
���������������ԣ��ܽ���������������+6�۵���������ӣ���������Ӻͱ����ӷ�Ӧ�������ᱵ������
��ͭ��Ũ���ᷴӦ��ͭ��������+2�۵�ͭ���ӣ����ᱻ��ԭ��+4�۵Ķ��������������ǿ�����ԣ���������������
�۵�A��KMnO4��Һû����ȫ��ɫ��˵�����������Ѿ���ȫ������ʢ��Na2SiO3��Һ���Թ��г��ְ�ɫ������˵��̼�ķǽ����Աȹ�ǿ��
��2���ٶ�����������Ԫ�صĻ��ϼ���+4�ۣ���������-2�۵���ԭ��
����������SO2ͨ��NaOH��Һ�У�����NaHSO3��
���������������ԣ��ܽ���������������+6�۵���������ӣ���������Ӻͱ����ӷ�Ӧ�������ᱵ������
���
�⣺��1����Ϊ�˱�֤ʵ���˳�����У�����װ��ҩƷ����װ�������Բ��ã����������������˷�ҩƷ������װ��©��Ӱ��ʵ��Ч����������������������װ�������ԣ�
�ʴ�Ϊ������װ�õ������ԣ�
��ͭ���ȵ�Ũ���ᷴӦ����Ӧ��CuԪ�صĻ��ϼ���0���ߵ�+2�ۣ�����ԭ��������Ϊ������������ͭ��ˮ����������������ԣ����������������ܷ���������ԭ��Ӧ����Ӧ�ķ���ʽΪCu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O������KMnO4��Һ��
�۶�����������Ԫ�صĻ��ϼ���+4�ۣ������м��̬���������������л�ԭ�ԣ����������ǿ�����ԣ����ӷ���ʽΪ5SO2+2MnO4-+2H2O�T2Mn2++5SO42-+4H+����A��KMnO4��Һû����ȫ��ɫ��˵�����������Ѿ���ȫ�����������˶�������Ϳ����Թ����η�Ӧ��������̼��ˮ��Ӧ����̼�ᣬ̼��Ϳ����Թ����η�Ӧ���������ɫ������˵��̼������ȡ���ᣬ��֤��̼������ǿ�ڹ������ԣ�
�ʴ�Ϊ��A������KMnO4��Һ����ɫ��Na2SiO3��Һ�г��ְ�ɫ������
��2���ٶ�����������Ԫ�صĻ��ϼ���+4�ۣ��������ԣ���������������H2S��Һ�����·�Ӧ�����ɻ�ɫ�������ʣ���ˮ����Ӧ�ķ���ʽΪ2H2S+SO2�T3S��+2H2O��
�ʴ�Ϊ��2H2S+SO2�T3S��+2H2O��
����������SO2ͨ��NaOH��Һ�У�����NaHSO3������ʽΪSO2+NaOH�TNaHSO3���ʴ�Ϊ��SO2+NaOH�TNaHSO3��
��BaCl2��Һ��������������ֳ����ݣ�һ�ݵμ���ˮ��Һ����ˮ�����������ӣ��������Ӿ��������ԣ��ܰѶ�������������+6�۵���������ӣ���������Ӻͱ����ӷ�Ӧ�������ᱵ��ɫ��������Ӧ�ķ���ʽΪBa2++SO2+Cl2+2H2O�TBaSO4��+4H++2Cl-����һ���еμӰ�ˮ�����������ˮ���������ᣬ������Ͱ�ˮ��Ӧ����������泥�������淋�����������Ӻ�����������ӣ�����������Ӻͱ����ӷ�Ӧ���������ᱵ������
�ʴ�Ϊ��
Ba2++SO2+Cl2+2H2O�TBaSO4��+4H++2Cl-��
�ʴ�Ϊ������װ�õ������ԣ�
��ͭ���ȵ�Ũ���ᷴӦ����Ӧ��CuԪ�صĻ��ϼ���0���ߵ�+2�ۣ�����ԭ��������Ϊ������������ͭ��ˮ����������������ԣ����������������ܷ���������ԭ��Ӧ����Ӧ�ķ���ʽΪCu+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
�۶�����������Ԫ�صĻ��ϼ���+4�ۣ������м��̬���������������л�ԭ�ԣ����������ǿ�����ԣ����ӷ���ʽΪ5SO2+2MnO4-+2H2O�T2Mn2++5SO42-+4H+����A��KMnO4��Һû����ȫ��ɫ��˵�����������Ѿ���ȫ�����������˶�������Ϳ����Թ����η�Ӧ��������̼��ˮ��Ӧ����̼�ᣬ̼��Ϳ����Թ����η�Ӧ���������ɫ������˵��̼������ȡ���ᣬ��֤��̼������ǿ�ڹ������ԣ�
�ʴ�Ϊ��A������KMnO4��Һ����ɫ��Na2SiO3��Һ�г��ְ�ɫ������
��2���ٶ�����������Ԫ�صĻ��ϼ���+4�ۣ��������ԣ���������������H2S��Һ�����·�Ӧ�����ɻ�ɫ�������ʣ���ˮ����Ӧ�ķ���ʽΪ2H2S+SO2�T3S��+2H2O��
�ʴ�Ϊ��2H2S+SO2�T3S��+2H2O��
����������SO2ͨ��NaOH��Һ�У�����NaHSO3������ʽΪSO2+NaOH�TNaHSO3���ʴ�Ϊ��SO2+NaOH�TNaHSO3��
��BaCl2��Һ��������������ֳ����ݣ�һ�ݵμ���ˮ��Һ����ˮ�����������ӣ��������Ӿ��������ԣ��ܰѶ�������������+6�۵���������ӣ���������Ӻͱ����ӷ�Ӧ�������ᱵ��ɫ��������Ӧ�ķ���ʽΪBa2++SO2+Cl2+2H2O�TBaSO4��+4H++2Cl-����һ���еμӰ�ˮ�����������ˮ���������ᣬ������Ͱ�ˮ��Ӧ����������泥�������淋�����������Ӻ�����������ӣ�����������Ӻͱ����ӷ�Ӧ���������ᱵ������
�ʴ�Ϊ��
| BaSO4 | BaSO3 |
���������⿼��ʵ�鷽������ƣ��漰��ѧ����������ʡ���װ�õ�����ȣ�Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ���������ѶȲ�������ʵ��ԭ���ǽ���Ĺؼ���ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��������Ũ�ȵĹ�ϵ������˵��������ǣ�������
| A��Ũ�Ⱦ�Ϊ0.1 mol/L��NH4Cl��Һ�Ͱ�ˮ�������Ϻ�c��NH4+��+c��NH3?H2O��=2c��Cl- �� |
| B��������ˮ�У�c��Cl- ����c��H+ ����c��OH- ����c��ClO- �� |
| C��pH=5.6��CH3COOH��CH3COONa�����Һ�У�c��CH3COO-����c��Na+����c��H+ ����c��OH- �� |
| D�������£�pH=2�������pH=12�İ�ˮ�������Ϻ�c��NH4+ ����c��Cl- ����c��OH- ����c��H+ �� |
��ͼʵ��װ�á�ѡ�õ��Լ���ʵ������У�����ȷ���ǣ�������
A��ʵ������װ��A��ȡ������ |
B����Bװ�����հ���������ֹ������ |
C����Cװ��ϡ��Ũ����C�� |
D����Dװ�ó�ȥCO2�е�HCl�� |
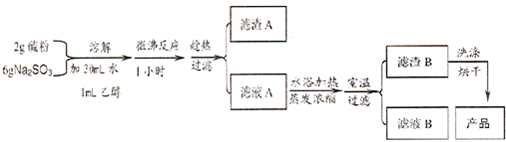
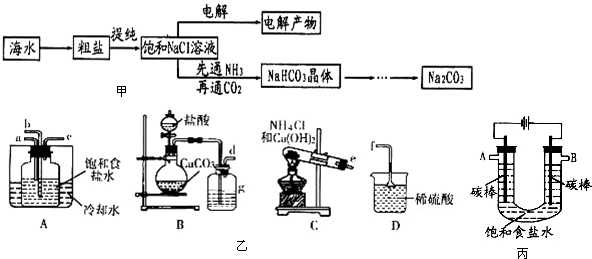

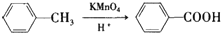
 ���������ױ�������
���������ױ�������