��Ŀ����
17����֪��ʯīΪ�缫�����CH3COONa��Һ����Ӧʽ���£�2CH3COONa+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$CH3CH3��+2CO2��+2NaOH+H2���������ͼ��ʾ�ж�����˵��������ȷ���ǣ�������
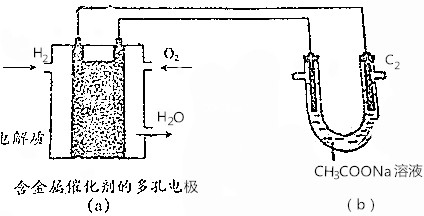
| A�� | ����a��������2.24LH2����b���أ�C1��������0.2mol CO2 | |
| B�� | ��a����ȼ�ϵ����������ӦʽΪO2+4e-+4H+�T2H2O | |
| C�� | ����b���ص������Һ�������ɣ��õ�NaOH���� | |
| D�� | ��b�����Ҳ�ʯī�缫��C2��Ϊ�������� |
���� A��״����֪��������������ʵ�����
B����a����ȼ�ϵ�����������õ��ӣ�������ԭ��Ӧ��
C������b���ط����ĵ缫��ӦΪ��2CH3COONa+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$CH3CH3��+2CO2��+2NaOH+H2�������Ե������Һ������������Һ����Һ�������ɣ��õ�NaOH���壻
D��ʯī�缫��C2����ԭ��صĸ���������
��� �⣺A��״����֪��������������ʵ�������������ת�Ƶ��ӵ����ʵ�������A����
B����a����ȼ�ϵ�����������õ��ӣ�������ԭ��Ӧ���缫��ӦʽΪ��O2+4e-+4H+�T2H2O����B��ȷ��
C������b���ط����ĵ缫��ӦΪ��2CH3COONa+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$CH3CH3��+2CO2��+2NaOH+H2�������Ե������Һ������������Һ��������Һ�������ɣ��õ�NaOH���壬��C��ȷ��
D��ʯī�缫��C2����ԭ��صĸ������������ԣ�b�����Ҳ�ʯī�缫��C2��Ϊ������������D��ȷ��
��ѡA��
���� ���⿼��绯ѧ��֪ʶ��Ϊ��Ƶ���㣬���ؿ���ѧ��ʶͼ��������ԭ���ԭ�������⣬��ȷ�����缫�Ϸ����ķ�Ӧ���缫��Ӧʽ����д�ǽⱾ��ؼ�����Ŀ�ѶȲ���

 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�| A�� | ������Ҵ���������Ӧ | |
| B�� | �Ҵ���Na��Ӧ | |
| C�� | �Ҵ���������Cu�����������·�Ӧ | |
| D�� | �����������������£�����������Ӧ |
| ��A | ��A | ��A | ��A | VA | ��A | VIIA | 0 | |
| 1 | �� | |||||||
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | |||||
| 4 | �� |
��2��Ԫ�آٺ͢�����γɵĻ��������ԭ�Ӹ�����Ϊ1��1�Ļ�����ĽṹʽΪH-O-O-H��ѧ������Ϊ���Լ��Ǽ��Լ�
Ԫ�آں͢�����γɵĻ��������ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽΪ

��3���õ���ʽ��ʾ�ݺ͢�ԭ�ӽ��Ϊ��������γɹ��̣�
 ��
����4��Ԫ�آܵ�����������ˮ�����ϡ��Һ��Cu������Ӧ�����ӷ���ʽ3Cu+8H++2NO3-�T3Cu2++2NO+4H2O��
��5��д��Ԫ�آݵ����ڳ�����Ϊ���壬ʵ���п�����NaOH��Һ���գ���Ӧ�����ӷ���ʽΪCl2+2OH-�T2Cl-+ClO-+2H2O��
| A�� | ��������ƽ����ҩƷʱ����ҩƷ������ƽ���� | |
| B�� | ����ʵ���У�Ҫ����ƿ�м����ʯ�����Ƭ���Է�ֹҺ�屩�� | |
| C�� | ��Ũ����ע��ʢˮ����Ͳ��ϡ�� | |
| D�� | ����������м�����������ʱ�������ʯ���� |
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
��2�������ϱ����ݣ��ж����з�Ӧ���ܳ�������D
A��CH3COOH+Na2CO3�TNaHCO3+CH3COONa
B��CH3COOH+NaCN�TCH3COONa+HCN
C��CO2+H2O+NaClO�TNaHCO3+HClO
D��CO2+H2O+2NaClO�TNa2CO3+2HClO
��3��Ҫ������ˮ�е�HClO��Ũ�ȣ�������ˮ�м���������NaHCO3����Na2CO3��NaHCO3������Ӧ�����ӷ���ʽΪCl2+HCO3-=Cl-+CO2��+HClO��
��4��Na2CO3��Һ��pH=11.6��ԭ���ǣ������ӷ���ʽ��ʾ����CO32-+H2O?HCO3-+OH-����Һ�и�����Ũ���ɴ�С��˳��Ϊc��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+��
��5��pH=11��NaOH��NaCN��Һ�У���ˮ�������c��H+��֮��Ϊ10-8��1
��6�������£���2amol/L��HCN��Һ��amol/L��NaOH��Һ�������ϣ���û��Һ��pH=8������Һ��c��HCN��-c��CN-��=2��10-6-10-8��mol/L��

��1���������ijɷ��м�������������Ӧ����Al2O3��д�����ܹ���Fe2O3��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ��Fe2O3+6H+�T2Fe3++3H2O��
��2�����������У�Ϊ��ȷ������Ĵ��ȣ�����������Ҫ������Һ��pH�ķ�Χ��3.2-3.8������������������������ʽ����ʱ��Һ��pH������
| ������ | Fe��OH��3 | Al��OH��3 | Fe��OH��2 | Mg��OH��2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 |
��4�����֤��ϴ�ӻ��ڳ��ϴ��ȡ�������һ��ϴ����Һ������1��2��Ba��NO3��2��Һ���������ְ�ɫ���ǣ���ʾ��ϴ����ȫ��
��5����֪����������Ϊa kg�����������Ʊ���������Ԫ�����25%�����յõ����������Ϊb kg����ԭ������������Ԫ����������Ϊ$\frac{14b}{15a}$�����������������ݼ��㲢����������ȱ�ʾ�����
| A�� | ���Է���Ӧ���Dz����ܷ����ķ�Ӧ���Է���Ӧ�����ܽϿ���еķ�Ӧ | |
| B�� | ���¸�ѹ�¿���ʹʯīת��Ϊ���ʯ���Է��Ļ�ѧ��Ӧ | |
| C�� | �������оݺ����о���϶��ɵĸ����оݣ������ʺ������еĹ��� | |
| D�� | ��ӦNH3��g��+HCl��g���TNH4Cl��s���ڵ��������Է����У�˵���÷�Ӧ�ġ�H��0 |
| A�� | C4H8��C5H10 | B�� | C3H8��C4H10 | ||
| C�� | C2H4O2��C3H6O2 | D�� | C6H5OH��C6H5CH2OH |

 ����Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ��ȡ����Ӧ��
����Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ��ȡ����Ӧ�� ��
�� ��
��