��Ŀ����
12��������ĿҪ��ش��������⣺�����£�Ũ��Ϊ0.1mol/L������������Һ��pH�����| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
��2�������ϱ����ݣ��ж����з�Ӧ���ܳ�������D
A��CH3COOH+Na2CO3�TNaHCO3+CH3COONa
B��CH3COOH+NaCN�TCH3COONa+HCN
C��CO2+H2O+NaClO�TNaHCO3+HClO
D��CO2+H2O+2NaClO�TNa2CO3+2HClO
��3��Ҫ������ˮ�е�HClO��Ũ�ȣ�������ˮ�м���������NaHCO3����Na2CO3��NaHCO3������Ӧ�����ӷ���ʽΪCl2+HCO3-=Cl-+CO2��+HClO��
��4��Na2CO3��Һ��pH=11.6��ԭ���ǣ������ӷ���ʽ��ʾ����CO32-+H2O?HCO3-+OH-����Һ�и�����Ũ���ɴ�С��˳��Ϊc��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+��
��5��pH=11��NaOH��NaCN��Һ�У���ˮ�������c��H+��֮��Ϊ10-8��1
��6�������£���2amol/L��HCN��Һ��amol/L��NaOH��Һ�������ϣ���û��Һ��pH=8������Һ��c��HCN��-c��CN-��=2��10-6-10-8��mol/L��
���� ��1�����������ˮ��̶�Խ����ͬŨ�ȵ�������Һ��pHԽ����������ӽ����������Խ��
��2��ǿ���ܺ������η�Ӧ���ɸ������
��3������HCl��H2CO3��HClO��HCO3-����ˮ��HCl�ܺ�̼���ơ�̼�����ƶ���Ӧ��HClO�ܺ�̼���Ʒ�Ӧ����̼�����Ʋ���Ӧ��
��4��̼������ǿ�������Σ�̼�����������ˮ���������������Ӷ�������ˮ��Һ�ʼ��ԣ����һ��ˮ��̶�ԶԶ���ڵڶ������ٽ�ϵ���غ��жϣ�
��5����������ˮ���룬���������ӵ��δٽ�ˮ���룬pH=11��NaOH��NaCN��Һ�У���ˮ�������c��H+���ֱ�Ϊ10-11 mol/L��10-3 mol/L��
��6�������£���2amol/L��HCN��Һ��amol/L��NaOH��Һ�������ϣ���Һ������Ϊ�����ʵ���Ũ�ȵ�HCN��NaCN����û��Һ��pH=8����Һ�д��ڵ���غ�������غ㣬���ݵ���غ�������غ��жϣ�
��� �⣺��1�����������ˮ��̶�Խ����ͬŨ�ȵ�������Һ��pHԽ����������ӽ����������Խ������ҺpH֪��CO32-��ˮ��̶������CO32-�������������ǿ���ʴ�Ϊ��CO32-��
��2���������Խǿ�����������ˮ��̶�Խ������ͬŨ�ȵ���������Һ��pHԽС������������ҺpH��С˳��֪�����ǿ��˳����CH3COOH��H2CO3��HClO��HCN��HCO3-��
����ǿ����ȡ����֪��ֻ��D�����ϣ�����D����
�ʴ�Ϊ��D��
��3������HCl��H2CO3��HClO��HCO3-����ˮ��HCl�ܺ�̼���ơ�̼�����ƶ���Ӧ��HClO�ܺ�̼���Ʒ�Ӧ����̼�����Ʋ���Ӧ�����Կ�����̼������Һʵ�֣����ӷ�Ӧ����ʽΪCl2+HCO3-=Cl-+CO2��+HClO��
�ʴ�Ϊ��NaHCO3��Cl2+HCO3-=Cl-+CO2��+HClO��
��4��̼������ǿ�������Σ�̼�����������ˮ���������������Ӷ�������ˮ��Һ�ʼ��ԣ�ˮ�����ӷ���ʽΪCO32-+H2O?HCO3-+OH-�����һ��ˮ��̶�ԶԶ���ڵڶ������ٽ�ϵ���غ��c��Na+����c��CO32-������Һ������Ũ�ȴ�С˳����c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��5����������ˮ���룬���������ӵ��δٽ�ˮ���룬pH=11��NaOH��NaCN��Һ�У���ˮ�������c��H+���ֱ�Ϊ10-11 mol/L��10-3 mol/L����������Һ��ˮ�������������Ũ��֮��=10-11 mol/L��10-3 mol/L=10-8��1���ʴ�Ϊ��10-8��1��
��6�������£���2amol/L��HCN��Һ��amol/L��NaOH��Һ�������ϣ���Һ������Ϊ�����ʵ���Ũ�ȵ�HCN��NaCN����û��Һ��pH=8����Һ�д��ڵ���غ�������غ㣬
���ݵ���غ��c��Na+��+c��H+��=c��OH-��+c��CN-��
���������غ��2c��Na+��=c��HCN��+c��CN-��
����c��HCN��-c��CN-��=2[c��OH-��-c��H+��]=2��10-6-10-8��mol/L��
�ʴ�Ϊ��2��10-6-10-8��mol/L��
���� ���⿼���������Һ�����жϡ�����ˮ�⡢ǿ����ȡ�����֪ʶ�㣬Ϊ��Ƶ���㣬���ؿ���ѧ������������������ȷ��ĵ���̶������������ˮ��̶ȹ�ϵ�ǽⱾ��ؼ���ע�⣺̼������Ӷ�Ӧ������̼��������Ӷ�����̼�ᣬΪ�״��㣮

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| A�� | ��������ǽ�̫����ת��Ϊ���� | |
| B�� | Ga��N��Ԫ�����ڱ��в�����ͬһ���� | |
| C�� | YAG������+3�� | |
| D�� | ��ͼ��N���뵼��Ϊ������P���뵼��Ϊ������������a����b |
| A�� | ��pH=7ʱ��c��Na+ ��=c��CH3 COO- ����c��H+ ��=c��OH- �� | |
| B�� | ��pH��7ʱ��c��CH3 COO- ����c��Na+ ����c��OH- ����c��H+ �� | |
| C�� | ��ǡ����ȫ�к�ʱ��c��Na+ ����c��CH3 COO- ����c��OH- ����c��H+ �� | |
| D�� | ������Һ��ʲô�Զ��й�ϵ��c��Na+ ��+c��H+ ��=c��CH3 COO- ��+c��OH- �� |
| A�� | ú�к��б��ͼױ��������ȸ�������ķ��������Ƿ������ | |
| B�� | ��C18�������������;������ѻ����Եõ����� | |
| C�� | ú�����л����������ɵĸ��ӻ���� | |
| D�� | ʯ�ͺ���C5��C11������������ͨ��ʯ�͵ķ�������� |
| A�� | HCl | B�� | NaOH | C�� | Na 2SO 4 | D�� | NaCl |
�������ͼ��ʾ�ж�����˵��������ȷ���ǣ�������
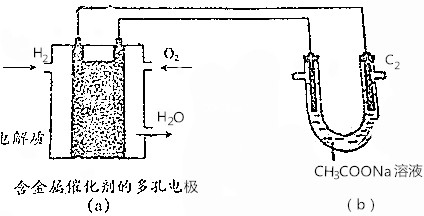
| A�� | ����a��������2.24LH2����b���أ�C1��������0.2mol CO2 | |
| B�� | ��a����ȼ�ϵ����������ӦʽΪO2+4e-+4H+�T2H2O | |
| C�� | ����b���ص������Һ�������ɣ��õ�NaOH���� | |
| D�� | ��b�����Ҳ�ʯī�缫��C2��Ϊ�������� |
| A�� | C2H6 | B�� | C3H6 | C�� | C3H8 | D�� | C4H8 |
| A�� | Va��Vbʱ��c ��CH3COOH����c ��CH3COO-����c ��K+�� | |
| B�� | Va=Vbʱ��c ��CH3COOH��+c ��H+����c ��OH-�� | |
| C�� | Va��Vbʱ��c ��CH3COO-����c ��K+����c ��OH-����c ��H+�� | |
| D�� | Va��Vb�����ʱ��c ��K+��+c ��H+��=c ��OH-��+c ��CH3COO-�� |
| A�� | ��״���£�5.6 L CO2������Na2O2��Ӧת�Ƶĵ�����Ϊ0.5 NA | |
| B�� | 78 g������̼̼˫������ĿΪ3 NA | |
| C�� | �����£�4.4gCO2��N2O������������е�ԭ����Ϊ0.3 NA | |
| D�� | 1 L 1 mol•L-1��CuSO4��Һ�к�NA��Cu2+ |