��Ŀ����
4���Ҷ���HSCH2CH2SH����һ��������ˮ�ľ�ϸ������Ʒ���۵�-41�棬�е� 144�棬���������ԣ�ʵ�����������1��2-��������Ϊԭ����ȡ�Ҷ����Ƶĺϳ�·�����£�
�Ʊ�װ����ͼ1��ʾ�����Ⱥͼг�װ�����ԣ���

�ش��������⣺
��1��ȡ��������������ƿ�У������Ҵ��������ܽ⣬�ټ���1��2-�������飮һ��ʱ����������������������������ܵ���������������ʹ������a��������ͨ��Һ©�����ŵ���ƽ��©��������ƿ��ѹ��������Һ˳�����£�
��2�����ˣ��õ������������ξ��壮����Һ�л����Ҵ��IJ���������
��3����ʹ��ͼ1��װ�ã��������������ξ��������NaOH��Һ����1.5Сʱ����ȴ���ټ���ϡH2SO4���ɵ��Ҷ���
�ټ���ϡH2SO4�����Ҷ��Ļ�ѧ����ʽΪNaSCH2CH2SNa+H2SO4=HSCH2CH2SH+Na2SO4��
�ڴӷ�Ӧ��Ļ�����з����Ҷ��IJ����Ƿ�Һ��
�۴�ʱʹ�ø�װ����һ������ȱ�ݣ��Ľ��Ĵ�ʩ���������հ�����װ�ã�
��4�������ϣ���ȡ9.4g�Ҷ���M=94g•mol-1����ͬʱ�����Ƶ�NaBr20.6g��
��5��NaBr��Na2SO4���ܽ�����¶ȵı仯��ͼ2��ʾ�������ӷ�Һ����ȡNa2SO4�ķ�������ʢ�з�Һ���ձ����ڱ�ˮԡ�У������������ˣ�ϴ�ӣ�
��6��Ϊ��֤���Ҷ����к���̼Ԫ�أ�ijͬѧȡ�������Ҷ����ȼ�գ��������ɵ�����ͨ�����ʯ��ˮ�У���ͬѧ��Ϊ����ʯ��ˮ����Ǽ���֤������ͬѧ�������Ǵ���ģ�������ȼ�ղ����к���SO2���壬Ҳ��ʹ����ʯ��ˮ����ǣ�
���� ��1�����������������������ã�����aͨ��֧�ܽ�����©���е���������a�У��Ӷ�����a��ѹǿ��
��2�����ܵ�Һ���������ķ������룻
��3����NaSCH2CH2SNa��H2SO4��Ӧ����HSCH2CH2SH��Na2SO4��
�ڻ������ܵ�Һ����÷�Һ�������룻
�۰����д̼�����ζ������ֱ���ſգ�
��4�����ݷ���ʽ֪��NaSCH2CH2SNa��HSCH2CH2SH��2NaBr֪���Ҷ���M=94g•mol-1����NaBr�����ʵ���֮��Ϊ1��2��
��5������ͼ֪���������ܽ�����¶ȱ仯�������������¶ȵĽ��ͣ����ܽ�ȱ仯�ϴ�
��6��������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ�
��� �⣺��1�����������������������ã��Ӷ�����Ӧ���ת���ʣ������������ʵIJ��ʣ�����aͨ��֧�ܽ�����©���е���������a�У��Ӷ�����a��ѹǿ������Һ˳�����£�
�ʴ�Ϊ������������ƽ��©��������ƿ��ѹ��������Һ˳�����£�
��2�����ܵ�Һ���������ķ������룬�����ܣ����Բ�������ķ������룬�ʴ�Ϊ������
��3����NaSCH2CH2SNa��H2SO4��Ӧ����HSCH2CH2SH��Na2SO4����Ӧ����ʽΪNaSCH2CH2SNa+H2SO4=HSCH2CH2SH+Na2SO4���ʴ�Ϊ��NaSCH2CH2SNa+H2SO4=HSCH2CH2SH+Na2SO4��
�ڻ������ܵ�Һ����÷�Һ�������룬���߲����ܣ����Բ��÷�Һ�������룬�ʴ�Ϊ����Һ��
�۰����д̼�����ζ������ֱ���ſգ�����Ӧ���������հ�����װ�ã��ʴ�Ϊ���������հ�����װ�ã�
��4�����ݷ���ʽ֪��NaSCH2CH2SNa��HSCH2CH2SH��2NaBr֪���Ҷ���M=94g•mol-1����NaBr�����ʵ���֮��Ϊ1��2��������NaBr������Ϊxg��
HSCH2CH2SH��2NaBr
94 206
9.4g x
94��206=9.4g��x
x=$\frac{9.4g}{94}��206$=20.6g��
�ʴ�Ϊ��20.6��
��5������ͼ֪���������ܽ�����¶ȱ仯�������������¶ȵĽ��ͣ����ܽ�ȱ仯�ϴ���������뷽��Ϊ����ʢ�з�Һ���ձ����ڱ�ˮԡ�У������������ˣ�ϴ�ӣ��ʴ�Ϊ����ʢ�з�Һ���ձ����ڱ�ˮԡ�У������������ˣ�ϴ�ӣ�
��6��������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ�Ҫ�ó���ʯ��ˮ���ն�����̼��Ӧ���ȳ�ȥ��������ĸ��ţ��ʴ�Ϊ��ȼ�ղ����к���SO2���壬Ҳ��ʹ����ʯ��ˮ����ǣ�
���� ���⿼�������Ʊ�ʵ�鷽����ƣ�Ϊ��Ƶ���㣬���ؿ��������ʵ���������������������������ȷʵ��ԭ�������������ǽⱾ��ؼ���֪���������������ü�ʵ�������������������Ԫ�ػ��������ʣ����ʼ���ʱҪ�ų��������ʵĸ��ţ���Ŀ�ѶȲ���


| A�� | 3��4��4-�������� | B�� | 3��3-����-2-�һ����� | ||
| C�� | 3��3��4-�������� | D�� | 3��3-����-4-�һ����� |
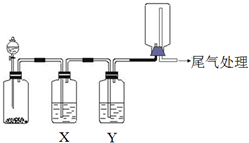 ������ͼװ�ÿ��Խ���ʵ�鲢�ܴﵽʵ��Ŀ���ǣ�������
������ͼװ�ÿ��Խ���ʵ�鲢�ܴﵽʵ��Ŀ���ǣ�������| ѡ�� | ʵ��Ŀ�� | X���Լ� | Y���Լ� |
| A | ��MnO2��Ũ������ȡ���ռ����������Cl2 | ����ʳ��ˮ | Ũ���� |
| B | ��Cu��ϡ������ȡ���ռ����������NO | ˮ | Ũ���� |
| C | ��֤��ʯ�뱥��ʳ��ˮ��Ӧ���ɵ���������ʲ��ռ� | CuSO4��Һ | KMnO4 ��Һ |
| D | CaCO3��ϡ������ȡ���ռ����������CO2 | ����NaHCO3��Һ | Ũ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
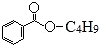 ������˵��������ǣ�������
������˵��������ǣ�������| A�� | X������ˮ | B�� | X�ķ���ʽΪC11H14O2 | ||
| C�� | ���Ϊ-C4H9��������3�� | D�� | X�ܷ����ӳɷ�Ӧ��ȡ����Ӧ |
| ѡ�� | A | B | C | D |
| ����ʽ | C3H8O | C3H8 | C7H16 | C8H10 |
| ������ | �����Ʒ�Ӧ | ���Ȼ��� | �����л���3��� | ���������ܵõ�3���������� |
| ͬ���칹����Ŀ | 2 | 3 | 2 | 3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���ᡢ���Ư�� | B�� | ���ᡢ�ռС�մ� | ||
| C�� | �������ơ���ʯ�ҡ������� | D�� | ���ᡢ��ʯ�ҡ������� |
 T��W��X��Y��Z��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ�������������������Ϣ���±���
T��W��X��Y��Z��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ�������������������Ϣ���±���| Ԫ�� | �����Ϣ |
| T | TԪ�ؿ��γ���Ȼ��Ӳ�����ĵ��� |
| W | W��Tͬ���ڣ�������һ��δ�ɶԵ��� |
| X | Xԭ�ӵĵ�һ�����������ĵ����ֱܷ��ǣ�I1=578kJ•mol��I2=1817kJ•mol-1��I3=2745kJ•mol-1��I4=11575kJ•mol-1 |
| Y | ���³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���� |
| Z | Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34������ |
��2����25�桢101kpa�£���֪13.5g��X���嵥����O2��������ȫȼ�պ�ָ���ԭ״̬������419kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ4Al��s��+3 O2��g��=2Al2O3��s����H=-3352kJ/mol��
��3����̬Yԭ���У�����ռ�ݵ�����ܲ����ΪM�����ܲ���е�ԭ�ӹ����Ϊ9��������Ϊ6��Y������WԪ�صĵ縺���ɴ�С˳��ΪF��O��S����Ԫ�ط������𣩣�
��4����֪Z�ľ����ṹ��ͼ��ʾ����֪Z���ܶ�Ϊ9.00g•cm3�����߳�Ϊ$\root{3}{4.72��1{0}^{-23}}$cm���ú���������ʽ�ӱ�ʾ����ZYO4�������Һ������YO42-�Ŀռ乹�����������壬����Yԭ�ӵ��ӻ����������sp3��Z�ĵ���������������е������Լ�������Ӧ�����ɳ����Z+HCl+O2�TZCl+HO2��HO2�������ᣩ������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԣ�����˵�����ʾ��ȷ����AD
A��O2��������
B��HO2����������
C��HO2�ڼ������ȶ�����
D��1molZ�μӷ�Ӧ��1mol���ӷ���ת�ƣ�
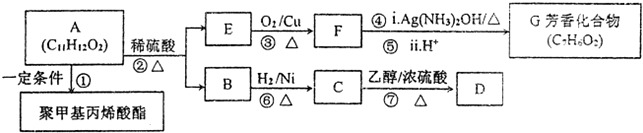
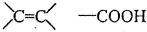 �������乬�������û�ѧ�Լ���������Ȼ�̼�������Ը��������Һ����̼��������Һ����ʯ���Լ�����
�������乬�������û�ѧ�Լ���������Ȼ�̼�������Ը��������Һ����̼��������Һ����ʯ���Լ����� ��
�� ��CH3��2CHCOOCH2CH3+H2O��
��CH3��2CHCOOCH2CH3+H2O��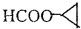 ��
��
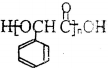 ��������д�м���Ľṹ��ʽ���ڡ������Ϸ����·�д��Ӧ�����������Լ���
��������д�м���Ľṹ��ʽ���ڡ������Ϸ����·�д��Ӧ�����������Լ���
 �й����ʵ�ת����ϵ��ͼ��ʾ�������������뷴Ӧ��������ȥ����A�dz����ķǽ������嵥�ʣ�F���Ϻ�ɫ�Ľ������ʣ�B��C�dz�����ǿ�ᣬD��G��I�dz��������壬D��I�����Ԫ����ͬ����D����Է���������I�Ĵ�16��E���������ɫҺ�壮��ش��������⣺
�й����ʵ�ת����ϵ��ͼ��ʾ�������������뷴Ӧ��������ȥ����A�dz����ķǽ������嵥�ʣ�F���Ϻ�ɫ�Ľ������ʣ�B��C�dz�����ǿ�ᣬD��G��I�dz��������壬D��I�����Ԫ����ͬ����D����Է���������I�Ĵ�16��E���������ɫҺ�壮��ش��������⣺ ��
��