��Ŀ����
16��Fe��Cu��Mn����Ԫ�صĵ��ʼ����������������������й㷺Ӧ�ã���l�� MnO2��һ�ֶ�ܲ��ϣ���ҵ�ϳ���Mn��NO3��2��6H2O������[CO��NH2��2]����������Ϊԭ���Ʊ������ط�����̼ԭ�ӵ��ӻ��������Ϊsp2��l mol���ط����к��еĦҼ���Ϊ7��6.02��1023��
��2��ijѧϰС�����ԷϾ��ڵ��Ϊԭ����ȡ�̣������������£�

����Ũ�����ܽ���������豣��ͨ�磬ԭ����MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O���û�ѧ����ʽ��ʾ����
��д��̼�����ڿ����������������������̵Ļ�ѧ����ʽ6MnCO3+O2$\frac{\underline{\;����\;}}{\;}$2Mn3O4+6CO2��
��3���ٻ�̬ͭԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1����Cuͬ������ԭ��������С�ĵڢ���Ԫ�أ����̬ԭ�Ӻ�����3��δ�ɶԵ��ӣ�
��ͼ1��Cu20�ľ����ṹ�������ı߳�Ϊacm����Cu2O���ܶ�Ϊ$\frac{288}{{a}^{3}{N}_{A}}$ g/cm3����NA��ʾ�����ӵ�������ֵ����
��4�����̷���FeS04•7H2O���Dz�Ѫ����ԭ�ϣ����治���ױ��ʣ������ʵ������̷��Ƿ���ȫ����ȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ�

�����ʻ�������Ϊ���壬�ᴿij�����������ۣ�����һЩ����Ӧ�����ʣ�����Ӧװ����ͼ2����֪��Fe��s��+5CO��g��?Fe��CO��5 ��H��0����ѹ�£�Fe�� CO��s���۵�ԼΪ-20.3�棬�е�Ϊ103.6�棬��Fe�� CO���ľ�������Ϊ���Ӿ��壮����ƽ���ƶ�ԭ������T1��T2��ԭ�����ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ���������T2ʱ��������ƽ�������ƶ����ʻ������ֽ⣬�����۲������Ҷˣ���T1��T2��
���� ��1�����ط�����Cԭ���γ�3���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ3��ÿ�������к���7���Ҽ���
��1����������������ڼ��������·�Ӧ������������Һ�к����Ȼ��̣�����̼��刺�����̼���̣�����������Mn3O4��������Ȼ�ԭ������Mn��
���������ж����壬Ҫ����ͨ�磬�����ж���
��̼�����ڿ���������ʱ���������뷴Ӧ��ͬʱ���ɶ�����̼��
��3����ͭԭ�Ӻ��������Ϊ29�������������ԭ����д�����Ų�ʽ����Cuͬ������ԭ��������С�ĵڢ���Ԫ��ΪFe�����̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d64s2��
�ڸ��ݾ�̯�����㾧���а�ɫ��ɫ����Ŀ����ϻ�ѧʽ�жϾ�����Cu��Oԭ����Ŀ���ټ��㾧�����������ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��4�����̷�����ʱ�������ӱ�����Ϊ�����ӣ������̷��Ƿ���ȫ���ʿ��Լ����Ƿ����������ӣ�
��Fe�� CO�����۷е�ͣ�Ӧ���ڷ��Ӿ��壻
���ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ���������T2ʱ��������ƽ�������ƶ����ʻ������ֽ⣬�����۲������Ҷˣ�
��� �⣺��1�����ط��ӵĽṹ��ʽΪ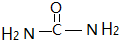 ���ӻ������ĿΪ3���������ط�����̼ԭ�ӵ��ӻ���ʽΪsp2�ӻ���ÿ�������к���7���Ҽ�������1mol�����к���7mol�Ҽ������еĦҼ���Ϊ7��6.02��1023��
���ӻ������ĿΪ3���������ط�����̼ԭ�ӵ��ӻ���ʽΪsp2�ӻ���ÿ�������к���7���Ҽ�������1mol�����к���7mol�Ҽ������еĦҼ���Ϊ7��6.02��1023��
�ʴ�Ϊ��sp2��7��6.02��1023��
��2����������������ڼ��������·�Ӧ������������Һ�к����Ȼ��̣�����̼��刺�����̼���̣�����������Mn3O4��������Ȼ�ԭ������Mn��
��Ũ������������̷�Ӧ�����������������ж����壬Ҫ����ͨ�磬�����ж�����Ӧ����ʽΪ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
��̼�����ڿ���������ʱ���������뷴Ӧ��ͬʱ���ɶ�����̼����Ӧ�ķ���ʽΪ6MnCO3+O2$\frac{\underline{\;����\;}}{\;}$2Mn3O4+6CO2��
�ʴ�Ϊ��6MnCO3+O2$\frac{\underline{\;����\;}}{\;}$2Mn3O4+6CO2��
��3����ͭԭ�Ӻ��������Ϊ29����������Ų�ʽΪ1s22s22p63s23p63d104s1����Cuͬ������ԭ��������С�ĵڢ���Ԫ��ΪFe�����̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d64s2��3d�ܼ���4��δ�ɶԵ��ӣ�
�ʴ�Ϊ��1s22s22p63s23p63d104s1��4��
�ھ����а�ɫ����ĿΪ1+8��$\frac{1}{8}$=2����ɫ����ĿΪ4���ʰ�ɫ��ΪOԭ�ӡ���ɫ��ΪCuԭ�ӣ���������Ϊ$\frac{2��144}{{N}_{A}}$g�����ܶ�Ϊ$\frac{2��144}{{N}_{A}}$g�£�a cm��3=$\frac{288}{{a}^{3}{N}_{A}}$g/cm3��
�ʴ�Ϊ��$\frac{288}{{a}^{3}{N}_{A}}$��
��4�����̷�����ʱ�������ӱ�����Ϊ�����ӣ�ʵ������̷��Ƿ���ȫ���ʷ���Ϊ��ȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ�
�ʴ�Ϊ��ȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ�
��Fe�� CO�����۷е�ͣ�Ӧ���ڷ��Ӿ��壻
���ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ���������T2ʱ��������ƽ�������ƶ����ʻ������ֽ⣬�����۲������Ҷˣ���T1��T2��
�ʴ�Ϊ�����Ӿ��壻���ۺ�һ����̼���ϳ��ʻ�����ʱ�ų����������������ںϳ��ӷ����ʻ��������ʻ������ӷ������ʲ����ڲ�������ˣ����ʻ������ӷ���������T2ʱ��������ƽ�������ƶ����ʻ������ֽ⣬�����۲������Ҷˣ���T1��T2��
���� ���⿼�黯ѧ�������̡�ʵ�鷽����ơ��������㡢ƽ���ƶ�ԭ��Ӧ�á��������������ʡ���������Ų����ӻ���ʽ�ȣ�ʵ����ƴ������Ŀ����Ҫѧ���߱���ʵ�Ļ��������Ӧ���������Ѷ��е�

| A�� | ����Һ�У�K+��Ca2+��Cl2��Br -���Դ������� | |
| B�� | ������Ca�� OH��2��Һ��Ӧ�����ӷ���ʽ��Ca2++OH-+HS03�TCaS03��+H20 | |
| C�� | ��FeCI3��Һ��Ӧ�����ӷ���ʽ��SO32-+2 Fe3++H20�TSO42-+2Fe2++2H+ | |
| D�� | ��ʹ��I2�ĵ�����Һ��ɫ��ȥ��˵��NaHSO3��Һ����Ư���� |
| A�� | ������Ϊ0.1NA ��N2 ��NH3 ������壬ԭ�Ӽ京�еĹ��õ��Ӷ���ĿΪ0.3NA | |
| B�� | 2 mol SO2 ��1 mol O2 ��һ�������³�ַ�Ӧ�����û������ķ���������2NA | |
| C�� | 1.5 mol NO2 ������ˮ��Ӧ��ת�Ƶĵ�����Ϊ1.5NA | |
| D�� | ���������£�������ΪNA ��CO��N2�����������Ϊ28 g |
| A�� | W������̼����������Ϊ1��2 | B�� | Wͬ���칹����5�� | ||
| C�� | ��������ײⶨW�����4���� | D�� | �ں˴Ź���������W������2���� |



 ��
�� ��
�� ��
�� ���������ַ��������Ƶð�ɫ��Fe��OH��2������
���������ַ��������Ƶð�ɫ��Fe��OH��2������ ���ɱ�ʾΪ
���ɱ�ʾΪ ��ij�л���
��ij�л��� �Ķ���ͬ���칹���У����ڷ��㴼��һ���У������������칹����������
�Ķ���ͬ���칹���У����ڷ��㴼��һ���У������������칹����������