��Ŀ����
9��ijͬѧ��10mol/L��Ũ��������250mL 1mol/L��ϡ���ᣬ�������й�ʵ�飮��ش��������⣺��1����Ҫ��ȡŨ����25.0mL��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ����������⣬�������õ��������н�ͷ�ι�250mL����ƿ��
��3��ȡ����ϡ����100mL����һ������������ַ�Ӧ����ȫ���ܽ�����ɵ������ڱ�״���µ����Ϊ0.896L����μӷ�Ӧ����������Ϊ2.24g����Ӧ�����Һ�м���100mLNaOH��Һǡ��ʹ��Ԫ����ȫ������������NaOH��Һ�����ʵ���Ũ��Ϊ1 mol/L��
���� ��1��������Һϡ�������������ʵ����ʵ������������ҪŨ���������
��2����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ������
��3�����ݷ���ʽ����μӷ�Ӧ��п���Ȼ�������ʵ���������m=nM����������������Ӧ�����Һ�м���100mLNaOH��Һǡ��ʹ��Ԫ����ȫ����������������ȫ��ת��Ϊ�Ȼ��ƣ�������ԭ�Ӹ����غ���㣮
��� �⣺��1������ҪŨ�������ΪV����������Һϡ�������������ʵ����ʵ�������ã�10moL/L��V=250mL��1mol/L�����V=25.0mL��
�ʴ�Ϊ��25.0��
��2������һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݣ��õ�����������Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ��������õ���������250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��3��Fe+2HCl=FeCl2+H2��
1mol 2mol 22.4L
x y 0.896L
���x=$\frac{0.896L}{22.4L}$=0.04mol��������������m=0.04mol��56g/mol=2.24g��
��Ӧ�����Һ�м���100mLNaOH��Һǡ��ʹ��Ԫ����ȫ����������������ȫ��ת��Ϊ�Ȼ��ƣ�������ԭ�Ӹ����غ�ã�n��HCl��=n��NaOH������100mL��1mol/L=100mL��C��NaOH��
���C��NaOH��=1mol/L��
�ʴ�Ϊ��1mol/L��
���� ���⿼����һ�����ʵ���Ũ����Һ�����Ƽ��йط���ʽ�ļ��㣬��ȷ����ԭ��������ʽ�и�����֮�����Ĺ�ϵ�ǽ���ؼ�����Ŀ�ѶȲ���

| A�� | ����Al�ܷų�H2����Һ�У�ClO-��HCO${\;}_{3}^{-}$��SO${\;}_{4}^{2-}$��NH${\;}_{4}^{+}$ | |
| B�� | pH=2����Һ�У�Fe2+��Na+��Mg2+��NO${\;}_{3}^{-}$ | |
| C�� | ��ʹKSCN��Һ������Һ�У�Na+��I-��NO${\;}_{3}^{-}$��HCO${\;}_{3}^{-}$ | |
| D�� | ��ˮ�������c��OH-��=1.0��10-13mol•L-1����Һ�У�Na+��Ba2+��Cl-��Br- |
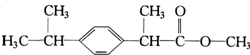 ij��ҩ����Ҫ�ɷ�X�ķ��ӽṹ��ͼ�������л���X��˵���д�����ǣ�������
ij��ҩ����Ҫ�ɷ�X�ķ��ӽṹ��ͼ�������л���X��˵���д�����ǣ���������X������ˮ���������л��ܼ�
��X�ܸ���ˮ��Ӧ
��X��ʹ���Ը��������Һ��ɫ
��X��ˮ������ܷ�����ȥ��Ӧ��
| A�� | �٢� | B�� | �ڢ� | C�� | �ڢ� | D�� | �٢� |

��֪��Fe3+��Fe2+��Cu2+ת��Ϊ��������ʱ����ʼ�����ͳ�����ȫʱ��pH���±���
| Fe3+ | Fe2+ | Cu2+ | |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| �������������ȫʱ��pH | 3.2 | 9.0 | 6.7 |
��2������3% H2O2֮ǰ�轫����Һ��ȴ����Ŀ���Ƿ�ֹ˫��ˮ�ֽ⣻����H2O2������Ӧ�����ӷ���ʽ2Fe2++H2O2+2H+=2Fe3++2H2O��
��3������2mol/L��ˮ����Һ��pHӦ��3.2��4.7��Χ�ڣ�
��4������������õ��ֵ���������������õ����Ƶ���������������ͬ������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ��裮���С�ϴ�ӡ���Ŀ����ϴȥ������������ӣ�
| ����ʽ | ��� | �ȷֽ��¶� | �۵� | �ܽ��� |
| CO��NH2��2•H2O2 | ��ɫ���� | 45�� | 75-85�� | ������ˮ���л��ܼ� |

��ش��������⣺
��1����Ӧ���ļ��ȷ�ʽ��ˮԡ���ȣ���Ӧ�¶���������ʵ��¶��½��У��¶Ȳ��ܹ��ߵ�ԭ�����¶ȹ��ߣ���Ʒ�ֽ⣬��ʹ�������������ͣ��¶�Ҳ���ܹ��͵�ԭ���¶ȹ��ͣ���Ӧ����̫�����ҷ�Ӧ��ϵ������Ҫ���Ĵ���������
��2��������ĸҺ�з����H2O2�����أ��ɲ��õIJ����Ǽ�ѹ���ᾧ��
��3���ɷ�������ȡ���������صķ����ǣ����ø�Ũ��˫��ˮˮ��Һ��������ˮ���ع����Ͻ��з�Ӧ��ˮ�ͷ�Ӧ��ͨ����̬����ȥ���õ�����Ĺ��������ز�Ʒ��
�Ƚϸɷ���ʪ�����ֹ��գ�����Ϊ�ɷ����յ��ŵ��ǣ����̶̣����ռ� �����һ�㼴�ɣ���
�ɷ����յ�ȱ���ǣ�˫��ˮŨ�ȸ߾���Ч��ͣ��豸���ӣ������������̣���Ʒ�ȶ��Բ��Ʒ��Ⱦ������ ��������㼴�ɣ���
ʪ�����յ��ŵ��ǣ���Ũ��˫��ˮ������Ч��ߣ��豸�����ڴﵽ���ܺĵͣ���Ʒ�ȶ��Ժã�ĸҺ��ѭ��ʹ�õ� ��������㼴�ɣ���
��4��ȷ��ȡ0.6000g��Ʒ��250mL��ƿ�У�����������ˮ�ܽ⣬�ټ�1mL 6mol•L-1 H2SO4����0.1000mol•L-1 KMnO4����Һ�ζ����յ�ʱ����20.00mL��������KMnO4��Һ����Ӧ�������Ʒ��CO��NH2��2•H2O2����������Ϊ78.3% �����������С�����һλ����
 ij��Һ�У����ܺ����±����������е�ij���֣�
ij��Һ�У����ܺ����±����������е�ij���֣�| ������ | Al3+��Mg2+��NH4+��Na+ |
| ������ | CO32-��SiO32-��[Al��OH��4]-��Cl- |
��1����X��NaOH��Һ��ԭ��Һ��һ�����е���������Cl-��BC�η�Ӧ�����ӷ���ʽΪAl��OH��3+OH-=[Al��OH��4]-��Al��OH��3+OH-=AlO2?+H2O��
��2����X�����ᣬ��ԭ��Һ��һ�����еĽ�����������Na+��AB�η�����Ӧ�������ӷ���ʽΪCO32-+2H+=H2O+CO2����OA�����ɳ��������ʵ���֮��Ϊ11��2��

