��Ŀ����
9����HCl����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.20g/cm3������1L1mol/L��ϡ���ᣮ�ش������й����⣬��������������裺
��1�����㣺����ȡ36.5%��Ũ��������Ϊ83.3������
��2����ȡ������Ͳ��ȡ����Ũ���Ტע�뵽250mL�ձ��У�
��3�������������²��������õ�����Ũ���ǡ�ƫ�ߡ�������ȡ����ǡ�ƫ�͡���
������Ͳ��ȡŨ�����������ˮϴ��Ͳ�������Һת������ƿ�У�ƫ�ߣ�
������ƿ��������������ˮ����ȣ�
��û�н�ϴ���ձ��Ͳ���������Һת������ƿ�У�ƫ�ͣ�
�ܶ��ݶ���ʱ����������ƿ�Ŀ̶��ߣ�ƫ�ߣ�
�ݶ���ҡ�Ⱥ�������ƿ��Һ����ڿ̶��ߣ��ּ�ˮ��ƫ�ͣ�
��δ��ȴ��ת�ƶ��ݣ�ƫ�ߣ�
����ϴ������ƿ��ƫ�ߣ�
���� ��1������c=$\frac{1000�Ѧ�}{M}$������Һ�����ʵ���Ũ�ȣ�������Һϡ���������ʵ����ʵ������������ҪŨ����������
��3���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=$\frac{n}{V}$������������
��� �⣺��1����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.20g/cm3�����ʵ���Ũ��C=$\frac{1000��1.2��36.5%}{36.5}$=12mol/L������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�V��12mol/L=1mol/L��1000mL�����V=83.3mL��
�ʴ�Ϊ��83.3��
��3��������Ͳ��ȡŨ�����������ˮϴ��Ͳ�������Һת������ƿ�У�������ȡŨ�������ƫ������ƫ�࣬��ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
������ƿ��������������ˮ�������ʵ����ʵ�������Һ���������Ӱ�죬��ҺŨ����ȣ�
�ʴ�Ϊ����ȣ�
��û�н�ϴ���ձ��Ͳ���������Һת������ƿ�У����²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�ܶ��ݶ���ʱ����������ƿ�Ŀ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ݶ���ҡ�Ⱥ�������ƿ��Һ����ڿ̶��ߣ��ּ�ˮ��������Һ���ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��δ��ȴ��ת�ƶ��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
����ϴ������ƿ�������������ʵ���ƫ����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע������ƿ���ѡ��ע���������ķ����ͼ��ɣ���Ŀ�ѶȲ���

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д����������繤ҵ����̼���Ƶķ�����·������N��Leblanc���������������£�
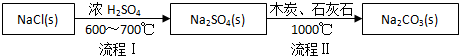
��1������I����һ������HCl�����̢�ķ�Ӧ�ֲ����У�a��Na2SO4+4C $\frac{\underline{\;1000��\;}}{\;}$Na2S+4CO����
b��Na2S��ʯ��ʯ�������ֽⷴӦ���ܷ�Ӧ����ʽ�ɱ�ʾΪNa2SO4+4C+CaCO3$\frac{\underline{\;1000��\;}}{\;}$Na2CO3+CaS+4CO����
��1862�꣬����ʱ������ά��Ernest Solvay���ð������̼���ƣ���Ӧԭ�����£�
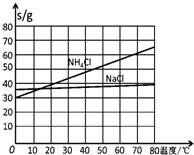
20��ʱһЩ������ˮ�е��ܽ��/g•��100gH2O��-1
| NaCl | NH4Cl | NaHCO3 | NH4HCO3 | Na2CO3 |
| 35.9 | 37.2 | 9.6 | 21.7 | 21.5 |
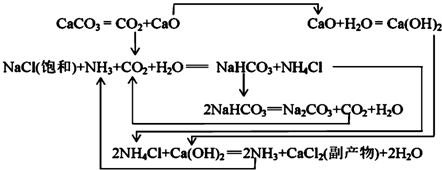
��3������NaCl��ҺͨNH3��CO2������NaHCO3��ԭ���У���Ӧ��ϵ��NaHCO3�ܽ����С����Ӧ����ˮ��NaHCO3��Է����������
���ҹ�����ר�Һ�°��о��������Ƽ���䷴Ӧԭ���Ͱ�����ƣ������ư����Ƽ����ϣ������ԭ�������ʣ�
��4����������������NaHCO3�����õ���Һ�м���NaCl���岢ͨ��NH3����0��10�棨���¶ȷ�Χ��������NH4Cl�����ѧʽ��
| A�� | ��ѧ��Ӧ�е������仯��ͨ������Ϊ�����ı仯 | |
| B�� | ���������������������Ȼ�茶����Ϸų��������÷�ӦΪ���ȷ�Ӧ | |
| C�� | ��Ӧ����1 molˮ������������Ϊ�к��� | |
| D�� | �ɻ�ѧ���������ų������������»�ѧ���γ������յ�����ʱ�������ȷ�Ӧ |
| A�� | 480mL����ƿ��7.68g����ͭ | B�� | 480mL����ƿ��12.0g���� | ||
| C�� | 500mL����ƿ��12.5g����ͭ | D�� | 500mL����ƿ��12.5g���� |
| A�� | 1 000 mL����ƿ��58.5 g NaCl | B�� | 980 mL����ƿ��57.3 g NaCl | ||
| C�� | 500 mL����ƿ��58.5 gNaCl | D�� | 1 000 mL����ƿ��117.0 g NaC1 |
| A�� | ���ۺ���ά�ض�����������������ˮ������������ | |
| B�� | ú��������Һ��������ú�ۺ����õ���Ҫ�����������������仯 | |
| C�� | �ع��ͷ���������Ӧ����뱥��ʳ��ˮ�����跢��Һ�����й������� | |
| D�� | ��������Һ�м���Ũ���������Һ���й������� |
 Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ�ı�ǩ��ijѧ������ʵ����������500mL��������ע��Һ��
Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ�ı�ǩ��ijѧ������ʵ����������500mL��������ע��Һ��
 ���ͬ���ڵ�����Ԫ�صĻ�̬ԭ�����������������ԭ����ͬ��Ԫ����K��Cu����Ԫ�ط��ţ�������һ�ֽ����ľ����ṹ��ͼ1��ʾ���þ����к��н���ԭ�ӵ���ĿΪ4��
���ͬ���ڵ�����Ԫ�صĻ�̬ԭ�����������������ԭ����ͬ��Ԫ����K��Cu����Ԫ�ط��ţ�������һ�ֽ����ľ����ṹ��ͼ1��ʾ���þ����к��н���ԭ�ӵ���ĿΪ4�� ������̼��ԭ��֮�乲�ۼ���c������ţ�
������̼��ԭ��֮�乲�ۼ���c������ţ� ������ʳ����Ҫ�ɷ֣����������»�ѧ���ʣ�
������ʳ����Ҫ�ɷ֣����������»�ѧ���ʣ�