��Ŀ����
2����1��ij�¶��£���ˮ��[H+]=2.0��l0-7mol•L-l�����ʱ��[OH-]=2.0��10-7 mol/L�����¶Ȳ��䣬����ϡ����ʹ[H+]=5.0��l0-7mol•L-l����[OH-]=8��10-8mol/L��
��ˮ�������[H+]=8��10-8mol/L��
��2����ˮ�м��������Ȼ�粒��壬NH3•H2O�ĵ���ƽ���������������ҡ��������ƶ�����ʱ��Һ$\frac{[O{H}^{-}]}{[N{H}_{3}•{H}_{2}O]}$��С�����������С�����䡱����
���� ��1�������¶ȸߵͣ���ˮ�ж�����c��H+��=c��OH-����
������Һ��c��OH-��=$\frac{{K}_{w}}{c��{H}^{+}��}$��������Һ��ˮ�������c��H+��������Һ��c��OH-����
��2��һˮ�ϰ���������ʣ���ˮ��Һ�д��ڵ���ƽ�⣬�����Ȼ�粒��壬����ͬ����ЧӦ������һˮ�ϰ����룻��Һ��c��NH3•H2O������c��OH-����С��
��� �⣺��1�������¶ȸߵͣ���ˮ�ж�����c��H+��=c��OH-��=2.0��10-7 mol/L��
������Һ��c��OH-��=$\frac{{K}_{w}}{c��{H}^{+}��}$=$\frac{2��1{0}^{-7}��2��1{0}^{-7}}{5.0��1{0}^{-7}}$mol/L=8��10-8mol/L��������Һ��ˮ�������c��H+��������Һ��c��OH-��Ϊ8��10-8mol/L��
�ʴ�Ϊ��2.0��10-7 mol/L��8��10-8mol/L��8��10-8mol/L��
��2��һˮ�ϰ���������ʣ���ˮ��Һ�д��ڵ���ƽ�⣬�����Ȼ�粒��壬����ͬ����ЧӦ������һˮ�ϰ����룬ƽ�������ƶ�����Һ��c��NH3•H2O������c��OH-����С������$\frac{[O{H}^{-}]}{[N{H}_{3}•{H}_{2}O]}$��С��
�ʴ�Ϊ������С��
���� ���⿼��������ʵĵ��뼰���ӻ��������㣬Ϊ��Ƶ���㣬���ؿ���ѧ����������������ע��������Һ��c��H+��������ˮ�������c��H+�����ѵ��Ǽ���������Һ��ˮ�������c��H+������Ŀ�ѶȲ���

| X | Y | |
| Z | W |
��1��Ԫ��Zλ�����ڱ��е�������VA�壮
��2��Z��W�γɵ���̬������ȶ�����ǿ����˳��ΪH2S��PH3�����ѧʽ��
��3��XW2�Ļ�ѧʽΪCS2��
��4��Y�����������Ļ�ѧʽΪHNO3��
| A�� | �������ơ����� | B�� | ���ᡢ��ˮ | ||
| C�� | �������ơ���ˮ | D�� | �������ơ�������̼ |
| A�� | ����12.5g����NaOHʱ����������������ϣ�NaOH���������� | |
| B�� | ѡ�õ�����ƿ������������ˮ | |
| C�� | ����ʱ���ӿ̶��� | |
| D�� | ����ҡ�Ⱥ�Һ���½����ּ�ˮ���̶��� |
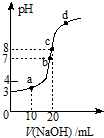 ����ʱ����0.10mol/L��NaOH��Һ�ζ�20.00mL 0.10mol/Lij һԪ��HA��Һ���õζ�������ͼ������˵������ȷ���ǣ�������
����ʱ����0.10mol/L��NaOH��Һ�ζ�20.00mL 0.10mol/Lij һԪ��HA��Һ���õζ�������ͼ������˵������ȷ���ǣ�������| A�� | a��b��c������ʾ��Һ����������ǿ����c���Ӧ����Һ | |
| B�� | ��c����Һ�У�c��H+��+c��HA��=c��OH-�� | |
| C�� | 25�棬HA�ĵ���ƽ�ⳣ��ԼΪ1.0��10-5 | |
| D�� | a��b��c��d�ĵ���ʾ��Һ��ˮ�ĵ���̶�������b���Ӧ����Һ |
| A�� | pH=1����Һ�У�NH4+��Na+��Fe3+��SO42- | |
| B�� | ������Һ�У�K+��Fe3+��Cl-��SO42- | |
| C�� | AlO2һ��Ũ��Ϊ0.1 mol/L����Һ�У�K+��Na+��HCO3-��SO42- | |
| D�� | Na2S��Һ�У�SO42-��K+��Cu2+��Cl- |