��Ŀ����
15������п�����̵��ң���Ҫ�ɷ�ΪZnO����������Fe2O3��CuO��SiO2��MnO�ȣ�Ϊԭ�Ͽ���������п���壨ZnC2O4•2H2O����
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Zn��OH��2 |
| ��ʼ������pH | 1.5 | 6.5 | 4.2 | 5.4 |
| ������ȫ��pH | 3.3 | 9.7 | 6.7 | 8.2 |
��1������A����Ҫ�ɷ�ΪSiO2��
��2�����̹����в���MnO��OH��2���������ӷ���ʽΪMn2++H2O2+H2O=MnO��OH��2��+2H+��
��3���ٳ���������Cu2+���ܱ���ȥ��ʱ����ZnO���Ʒ�ӦҺpH�ķ�ΧΪ3.3��5.4��
�����������г������ͭ��˳���ܵߵ�����������ʻ��С����ԭ�����ȼ���ZnS�ὫFe3+��ԭΪFe2+��ʹ��Ԫ�����Գ�ȥ��
��4�����������̲���Na2C2O4���棨NH4��2C2O4��������п���壬�����ļ��Ϸ�ʽ���ڽ����£���Na2C2O4�������뵽ZnCl2��Һ�У�

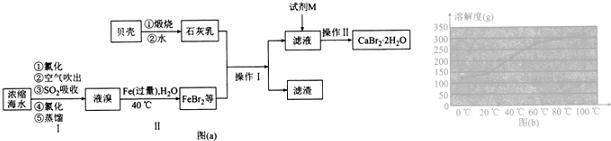
��5��������п������ȷֽ�ɵõ�һ�����ײ��ϣ����ȹ����й�����������¶ȵı仯����ͼ��ʾ��300�桫460�淶Χ�ڣ�������Ӧ�Ļ�ѧ����ʽΪZnC2O4$\frac{\underline{\;\;��\;\;}}{\;}$ZnO+CO��+CO2����
���� ���������ʱSiO2���ܽ⣬���˷��룬����AΪSiO2����Һ�м������������г��̣���ͨ��������ҺpH��ʹFe3+ת��ΪFe��OH��3���������˷��룬��Һ���ټ���ZnS��Cu2+ת��ΪCuS���������˳�ȥ����Һ��ע��Ϊ�Ȼ�п���������淋õ�����п���壬���յ���Һ�к����Ȼ�淋ȣ�
��1���ɷ�����֪������AΪ�������裬����BΪ����������
��2�����̹����в���MnO��OH��2���������ݵ���غ�Ӧ�����������ɣ�
��3���ٳ���������Cu2+���ܱ���ȥ��ʱ����ZnO����pHʹ�����ӳ�����ȫ����Zn2+���ܳ�����
��ZnS�ὫFe3+��ԭΪFe2+��ʹ��Ԫ�����Գ�ȥ��
��4����Na2C2O4���棨NH4��2C2O4��������п���壬���ɲ���識����Ȼ�п��Һ�����ɲ���п������
��5��ZnC2O4•2H2O�����е�ZnC2O4��������Ϊ$\frac{153}{153+36}$��100%=80.95%����A����ȫʧȥ�ᾧˮ����ѧʽΪZnC2O4������B��ΪZnO�����������ռ�е���������Ϊ$\frac{81}{153+36}$��100%=42.86%����B���������ΪZnO�����ԭ���غ��֪�����ɵ����ʵ�����CO��CO2��
��� �⣺���������ʱSiO2���ܽ⣬���˷��룬����AΪSiO2����Һ�м������������г��̣���ͨ��������ҺpH��ʹFe3+ת��ΪFe��OH��3���������˷��룬��Һ���ټ���ZnS��Cu2+ת��ΪCuS���������˳�ȥ����Һ��ע��Ϊ�Ȼ�п���������淋õ�����п���壬���յ���Һ�к����Ȼ�淋ȣ�
��1���ɷ�����֪������AΪSiO2���ʴ�Ϊ��SiO2��
��2�����̹����в���MnO��OH��2���������ݵ���غ�Ӧ�����������ɣ���Ӧ���ӷ���ʽΪ��Mn2++H2O2+H2O=MnO��OH��2��+2H+��
�ʴ�Ϊ��Mn2++H2O2+H2O=MnO��OH��2��+2H+��
��3���ٳ���������Cu2+���ܱ���ȥ��ʱ����ZnO����pHʹ�����ӳ�����ȫ����Zn2+���ܳ������ʿ��Ʒ�ӦҺpH�ķ�ΧΪ3.3��5.4��
�ʴ�Ϊ��3.3��5.4��
�ڳ������ͭ��˳���ܵߵ�����������ʻ��С����ԭ���ǣ��ȼ���ZnS�ὫFe3+��ԭΪFe2+��ʹ��Ԫ�����Գ�ȥ��
�ʴ�Ϊ���ȼ���ZnS�ὫFe3+��ԭΪFe2+��ʹ��Ԫ�����Գ�ȥ��
��4�����������̲���Na2C2O4���棨NH4��2C2O4��������п���壬�����ļ��Ϸ�ʽ�ǣ��ڽ����£���Na2C2O4�������뵽ZnCl2��Һ�У�
�ʴ�Ϊ���ڽ����£���Na2C2O4�������뵽ZnCl2��Һ�У�
��5��ZnC2O4•2H2O�����е�ZnC2O4��������Ϊ$\frac{153}{153+36}$��100%=80.95%����A����ȫʧȥ�ᾧˮ����ѧʽΪZnC2O4������B��ΪZnO�����������ռ�е���������Ϊ$\frac{81}{153+36}$��100%=42.86%����B���������ΪZnO�����ԭ���غ��֪�����ɵ����ʵ�����CO��CO2��300�桫460�淶Χ�ڣ�������Ӧ�Ļ�ѧ����ʽΪ��ZnC2O4$\frac{\underline{\;\;��\;\;}}{\;}$ZnO+CO��+CO2����
�ʴ�Ϊ��ZnC2O4$\frac{\underline{\;\;��\;\;}}{\;}$ZnO+CO��+CO2����
���� ���⿼�������Ʊ��������̣��ؼ��ǶԹ������̵����⣬�漰İ������ʽ��д�����ʵķ����ᴿ���������Ŀ������������ѧ����ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�Ǹ߿�������Ŀ����������Ԫ�ػ��������ʣ���Ŀ�Ѷ��еȣ�

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�| A�� | ��ȡ8.0g����ͭ������500mLˮ | |
| B�� | ��ȡ12.0g�������500mL��Һ | |
| C�� | ����ҡ�Ⱥ�Һ���½���Ӧ��ˮ����Һ����͵��������ƽ | |
| D�� | ����ʱ���ӿ���ʹ������ҺŨ��ƫС |
| A�� | n=2 | |
| B�� | ��һ�δ�ƽ��ʱ����Ӧ���ĵ�AΪ0.7 mol | |
| C�� | ���뵪����Ӧ���ʼӿ죬ƽ�������ƶ� | |
| D�� | ����Ӧ���� |
 �������̶������Ϊ1L�ܱ��������Բ�ͬ����̼����[n��H2����n��CO2��]����H2��CO2����һ�������·�����Ӧ��2CO2��g��+6H2��g��?C2H4��g��+4H2O��g����H��CO2��ƽ��ת����a��CO2�����¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
�������̶������Ϊ1L�ܱ��������Բ�ͬ����̼����[n��H2����n��CO2��]����H2��CO2����һ�������·�����Ӧ��2CO2��g��+6H2��g��?C2H4��g��+4H2O��g����H��CO2��ƽ��ת����a��CO2�����¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �÷�Ӧ�ڸ������Է����� | |
| B�� | ��̼�ȣ�X��2.0 | |
| C�� | ����ʼʱ��CO2��H2��Ũ�ȷֱַ�Ϊ0.5mol/L��1.0mol/L����ɵ�P�㣬��Ӧ�¶ȵ�ƽ�ⳣ����ֵΪ512 | |
| D�� | ����P��״̬�������У���2��4��1��4�ı����ٳ���CO2��H2��C2H4��H2O���ٴ�ƽ���a��CO2����С |