��Ŀ����
18�����ѾƳ���Na2S2O5��������������1��1.90g Na2S2O5����ܻ�ԭ224mLO2����״������
��2��0.5mol Na2S2O5�ܽ���ˮ���1L��Һ������ҺpH=4.5����Һ�в�����Ũ������Һ����Ա仯��ͼ��ʾ��
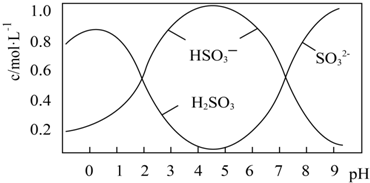
д��Na2S2O5�ܽ���ˮ�Ļ�ѧ����ʽNa2S2O5+H2O=2NaHSO3������ҺpHС��1����Һ��H2SO3��Ũ�ȱ�С����ԭ�������������ȶ����ֽ����ɶ������������ᱻ������
��֪��Ksp=1��10-10��Ksp=5��10-7���Ѳ��ֱ����������ĸ���ҺpH��Ϊ10������Һ�еμ�BaCl2ʹSO42-������ȫ����ʱ��Һ��c��SO32-����0.05mol•L-1��
��3�����Ѿ���Ʒ�п��������IJ������ⶨ����֪��SO2+I2+2H2O�TH2SO4+2HI����ȷ��ȡ100.00mL���Ѿ���Ʒ��������������������ɷ֣�ȡ�������ƿ�У��μ�����������Һ�������ʵ���Ũ��Ϊ0.0225mol•L-1��I2��Һ�ζ����յ㣬���ı�I2��Һ16.02mL���ظ����ϲ��������ı�I2��Һ15.98mL���������Ѿ���Ʒ�п��������IJ����� ����λ��mg•L-1����SO2���㣬�����������̣���
���� ��1��������ԭ��Ӧ��ת�Ƶ�����ȣ���ϵ����غ���㣻
��2������ҺpH=4.5����Һ�����ԣ���ͼ��֪����������HSO3-����ҺpHС��1����Һ��H2SO3��Ũ�ȱ�С�������������ᱻ������ֽ��йأ�Ksp[BaSO4]=c��Ba2+��•c��SO42-�����ɼ������Ҫc��Ba2+���������������Ũ��c��SO32-����
��3������Ϣ��֪�����ı�I2��Һ�����Ϊ$\frac{15.98mL+16.02mL}{2}$=16.0mL�����SO2+I2+2H2O�TH2SO4+2HI�����������������Դ������
��� �⣺��1�������������ΪxmL���ɵ����غ��֪��$\frac{1.90}{190g/mol}$��2����6-4��=$\frac{x��1{0}^{-3}L}{22.4L/mol}$��2����2-0�������x=224��
�ʴ�Ϊ��224��
��2������ҺpH=4.5����Һ�����ԣ���ͼ��֪����������HSO3-����Na2S2O5�ܽ���ˮ�Ļ�ѧ����ʽΪNa2S2O5+H2O=2NaHSO3������ҺpHС��1����Һ��H2SO3��Ũ�ȱ�С����ԭ�������������ȶ����ֽ����ɶ������������ᱻ��������Ksp[BaSO4]=c��Ba2+��•c��SO42-������֪��Ҫc��Ba2+��=$\frac{1��1{0}^{-10}}{1��1{0}^{-5}}$=10-5mol•L-1������Һ��SO32-�����Ũ��c��SO32-��=$\frac{5��1{0}^{-7}}{1{0}^{-5}}$=0.05mol•L-1��
�ʴ�Ϊ��Na2S2O5+H2O=2NaHSO3��������ȶ����ֽ����ɶ������������ᱻ������0.05��
��3������Ϣ��֪�����ı�I2��Һ�����Ϊ$\frac{15.98mL+16.02mL}{2}$=16.0mL������I2�����ʵ���Ϊ16.0��10-3L��0.0225mol•L-1=3.6��10-4mol�����ݷ�ӦSO2+I2+2H2O=H2SO4+2HI����֪������������ʵ���Ϊ3.6��10-4mol��SO2������Ϊ64g/mol��3.6��10-4mol=23.04mg���������Ѿ���Ʒ�п��������IJ�����Ϊ$\frac{23.04mg}{0.1L}$=230.4mg•L-1��
�����Ѿ���Ʒ�п��������IJ�����Ϊ230.4mg•L-1��
���� ���⿼���ﺬ�����㡢������ԭ��Ӧ���㼰ͼ�������Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ʵ����Ĺ�ϵ��Ϊ���Ĺؼ������ط�������������Ŀ��飬��Ŀ�ѶȲ���

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
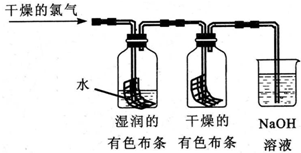
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�������ͭ˿����Ũ���ᣬ���ȣ�
������������ɫ����������ʱ�����ͭ˿��ֹͣ���ȣ�
����ȴ�ӷ�Ӧ��Ļ�����з������ɫ������ϴ�������ﱸ�ã�
��1������������������SO2��
��2��������У����ӷ�Ӧ��Ļ�����з������ɫ�������IJ����ǽ���Ӧ��Ļ���ﵹ��װ����ˮ���ձ��У���ȴ����ˣ�
��3����ͬѧ�����ɫ������CuO������������£�
�������ף�������Cu2+�ķ����ǣ�����Һ�еμ�K4[Fe��CN��6]��Һ�����������ɫ������֤����Cu2+��
�ٽ�CuO����ϡ�����У�һ��ʱ���δ�����������ٵμ�K4[Fe��CN��6]��Һ���������ɫ������
�ڽ���ɫ��������ϡ�����У�һ��ʱ��μ�K4[Fe��CN��6]��Һ��δ�����ɫ������
�ɸü���������ý����Ǻ�ɫ�����в�����CuO��
��4���ٴμ��裬��ɫ������ͭ�����ʵ�����£�
| ʵ��װ�� | ���� |
 | 1��A�Թ��к�ɫ�������ܽ� 2��A�Թ����Ϸ����ֺ���ɫ���� 3��B�Թ��г��ְ�ɫ���� |
�ڲ�������ɫ����Ļ�ѧ����ʽ��2NO+O2�T2NO2��
����ȷ�Ϻ�ɫ�����к���SԪ�ص�������B�Թ��г��ְ�ɫ��������Ӧ�����ӷ���ʽ��NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+��
��Ϊȷ�Ϻ�ɫ�����ǡ�ͭ�������������е�ʵ����ȡ��ȴ��Aװ���Թ��е���Һ���μ�K4[Fe��CN��6]��Һ�����������ɫ������֤����Cu2+��˵����ɫ������ͭ�����
��5������ʵ��˵������ɫ�����д���ͭ�������һ��ʵ���֤����ɫ������CuS��Cu2S�Ļ�������ɫ��������Ũ�����м���һ��ʱ������ܽ⣬����CuS�ܽ�Ļ�ѧ����ʽΪCuS+4H2SO4��Ũ���TCuSO4+4SO2��+4H2O��
| A�� | 100g•mol-1 | B�� | 108g•mol-1 | C�� | 55g•mol-1 | D�� | 96g•mol-1 |
 1807�껯ѧ�Ҵ�ά�õ���������������Ƶ��ƣ�4NaOH$\frac{\underline{\;ͨ��\;}}{\;}$4Na+O2��+2H2O��������•����������������������������Ҳ�Ƶ��ƣ���Ӧԭ��Ϊ��3Fe+4NaOH$\frac{\underline{\;1100��\;}}{\;}$Fe3O4+2H2��+4Na���������й�˵����ȷ���ǣ�������
1807�껯ѧ�Ҵ�ά�õ���������������Ƶ��ƣ�4NaOH$\frac{\underline{\;ͨ��\;}}{\;}$4Na+O2��+2H2O��������•����������������������������Ҳ�Ƶ��ƣ���Ӧԭ��Ϊ��3Fe+4NaOH$\frac{\underline{\;1100��\;}}{\;}$Fe3O4+2H2��+4Na���������й�˵����ȷ���ǣ�������| A�� | ������������������ƣ����������ĵ缫��ӦΪ��2OH--2e-�TH2��+O2�� | |
| B�� | ��•�����˷�����֤�����Ļ�ԭ�Ա���ǿ | |
| C�� | ���ô�ά�����•�����˷��Ƶõ������ƣ�������Ӧ����ת�Ƶĵ�������ͬ | |
| D�� | ��������Ȼ������Ƶĵ����У�����ͼ����ʯīΪ��������Ϊ���� |
| A�� | ͬһ�ܲ��px��py��pz�������������ͬ | |
| B�� | 3d3��ʾ3d�ܼ���3����� | |
| C�� | p�������������һ������s����������� | |
| D�� | ���������������˶��ĵ���������� |



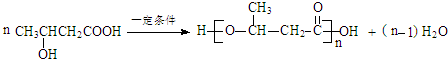 ����
���� ��
��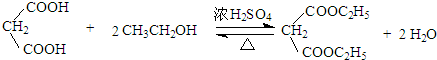 ��
��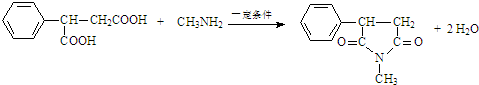 ��
��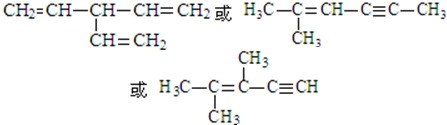 ��
��

 2SO2��g��+O2��g���T2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol��
2SO2��g��+O2��g���T2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol��