��Ŀ����
13����֪Cl2+H2O?HCl+HClO��Ϊ��̽��HClO��Ư���ԣ�ijͬѧ�������ͼ��ʾ��ʵ�飮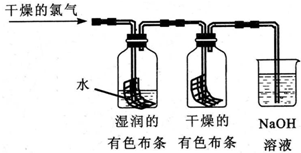
��1��ͨ��������������ƿ��ʪ�����ɫ�����ܿ���ɫ�����������ɫ����û����ɫ����˼�ͬѧ�ó����ۣ����������û��Ư���ԣ���ʪ��������Ư���ԣ�
��2��ϸ�ĵ���ͬѧ���֣�һ��ʱ���������ɫ����Ҳ��ɫ�ˣ���������ϸ˼������Ϊ�������ȱ�ݣ�Ӧ���������ģ���ʪ��IJ�������IJ���֮������һ������װ�ã�
��3����ͬѧ������ͬѧ�ķ����ĺ����ˡ�������ε���ɫ�����ϣ�������ɫ��������ɫ����һʵ�飬��ͬѧ���ȷ����HClOʹʪ�����ɫ������ɫ������ͬѧ��Ϊ���Dz���ȷ��HClOʹʪ�����ɫ������ɫ������Ϊ��Ӧ���ӵ�ʵ���Ǵ���������Һ����������Һ�μӵ���ɫ�����ϣ�����ɫ��������ɫ����֤��HClO��Ư���ԣ�
���� ��֪Cl2+H2O?HCl+HClO����������Ư��������Ϊ����ˮ��Ӧ���ɵ�HClO����Ư���ԣ����Ը����Cl2����ʹ��ɫ������ɫ��
��1��ͨ��������������ƿ��ʪ�����ɫ�����ܿ���ɫ�����������ɫ����û����ɫ��˵��������Ư���ԣ�
��2��ϸ�ĵ���ͬѧ���֣�һ��ʱ���������ɫ����Ҳ��ɫ�ˣ�˵��ˮ�������뷢����Ӧ�����˴����ᣬ��Ҫ�ڸ������ɫ����ǰ���Ӹ���װ�ã�
��3����ͬѧ֤������������Ư���ԣ�������ˮ��Ӧ���ɴ���������ᣬ��Һ�д��ڴ���������Ӷ����ܶ�����Ư���ԣ�������ε���ɫ�����ϣ�������ɫ��������ɫ˵������HCl��Ư���ԣ�������������Һ���뵽��ɫ��������ɫ˵�����Ǵ����������������ã��Ӷ�֤��Ư�����Ǵ���������ã�
��� �⣺��1��ͨ��������������ƿ��ʪ�����ɫ�����ܿ���ɫ�����������ɫ����û����ɫ����˼�ͬѧ�ó����ۣ����������û��Ư���ԣ���ʪ��������Ư���ԣ�
�ʴ�Ϊ�����������û��Ư���ԣ���ʪ��������Ư���ԣ�
��2��ϸ�ĵ���ͬѧ���֣�һ��ʱ���������ɫ����Ҳ��ɫ�ˣ���������ϸ˼������Ϊ�������ȱ�ݣ�ˮ����������������������ɫ�����ļ���ƿ��������ˮ��Ӧ��������ʹ��������Ư�����ã�������ɫ��װ��ӦӦ���������ģ���ʪ��IJ�������IJ���֮������һ������װ�ã�
�ʴ�Ϊ����ʪ��IJ�������IJ���֮������һ������װ�ã�
��3����ͬѧʵ�������֤������������Ư���ԣ�ʪ�����������Ư���ԣ�������ˮ��Ӧ���ɴ���������ᣬ��Һ�д��ڴ���������ӣ��������ɵ�HCl��HClO��ClO-�����ܶ�����Ư���ԣ�������ε���ɫ�����ϣ�������ɫ��������ɫ˵������HCl��Ư���ԣ�������������Һ���뵽��ɫ��������ɫ˵�����Ǵ����������������ã��Ӷ�֤��Ư�����Ǵ���������ã�������ӵ�ʵ���ǣ�����������Һ����������Һ�μӵ���ɫ�����ϣ�����ɫ��������ɫ����֤��HClO��Ư���ԣ�
�ʴ�Ϊ������������Һ����������Һ�μӵ���ɫ�����ϣ�����ɫ��������ɫ����֤��HClO��Ư���ԣ�
���� ���⿼�����������仯�������ʷ�������Ӧ���������Ӧ�ã���Ҫ����֤������Ư���Ե�ʵ����ƣ���Ŀ�Ѷ��еȣ�

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�| A�� | Fe��Al��Cu���Էֱ����û�����ֱ�Ӽ��ȷ��͵�ⷨұ���õ� | |
| B�� | ���ơ��������Ƚ���Ԫ�ص�������Ѥ������ɫ��������������� | |
| C�� | ʯ���ѽ⡢ú����������ˮ��þ��������ѧ�仯 | |
| D�� | ��ҵ��Ϊ�˼ӿ���뽺���еĵ�������ʣ�������������ʩ�ӵ糡��ʹ�������������Ĥ�������ƶ����ò�����Ӧ�ý���ĵ�Ӿԭ�� |
| A�� | KClO3��MnO2�����ȣ� | B�� | KMnO4�����ȣ� | C�� | H2O2��MnO2�� | D�� | HgO�����ȣ� |
| A�� | ��ʼ����������ò��������� | |
| B�� | ��ȥ���������ʺ���Һ���������ڼ���Ũ�� | |
| C�� | ��������ʣ������Һ��ʱ��ֹͣ���ȣ��������Ƚ�Һ������ | |
| D�� | ���Ƶþ���ת�Ƶ����ƹ��������ô���ˮ����ϴ�� |
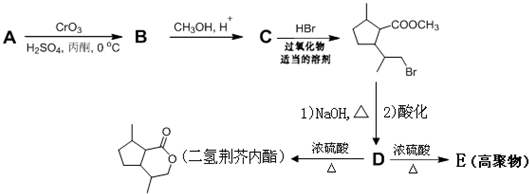
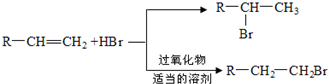
 ��D�к��еĹ����ŵ������Ȼ����ǻ���
��D�к��еĹ����ŵ������Ȼ����ǻ���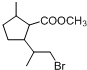 +2NaOH$\stackrel{��}{��}$
+2NaOH$\stackrel{��}{��}$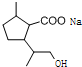 +CH3OH+NaBr��
+CH3OH+NaBr��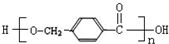 ����·�߲�ȫ����
����·�߲�ȫ����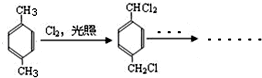
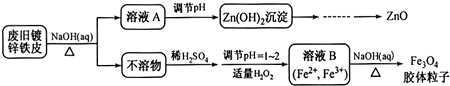
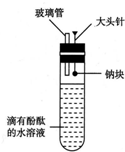 �����������ͼ��ʾ��ʵ��װ�ã�Ŀ����������ˮ��Ӧ��ʵ�鲢��֤��
�����������ͼ��ʾ��ʵ��װ�ã�Ŀ����������ˮ��Ӧ��ʵ�鲢��֤��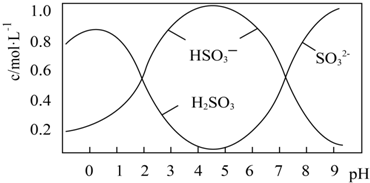
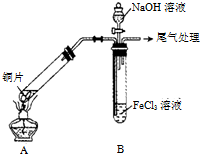 ijУ��ѧ��ȤС��̽��SO2��FeCl3��Һ�ķ�Ӧ������װ����ͼ��ʾ���г���������ȥ����
ijУ��ѧ��ȤС��̽��SO2��FeCl3��Һ�ķ�Ӧ������װ����ͼ��ʾ���г���������ȥ����