��Ŀ����
9������ͭ��ȡ��M��ͨ�����·�Ӧʵ��ͭ���ӵĸ�������ͼ1����
��1��X������ˮ���������л��ܼ����侧������Ϊ���Ӿ��壮
��2��X����sp2�ӻ���sp3�ӻ���ԭ�ӵĵ�һ�������ɴ�С˳��ΪN��O��C��
��3��������Ӧ�ж��Ѻ����ɵĻ�ѧ����be������ţ���
a�����Ӽ� b����λ�� c�������� d�����»��� e�����ۼ�
��4��M��W�����ӽṹ��ͼ2����ȣ�M��ˮ����С��������Cu2+����ȡ��Mˮ����С����Ҫԭ����M���γɷ����������ʹ�ܽ�ȼ�С��

��5������ͭ���壬�׳����������������д��£�����ⶾ�ȹ�Ч��ȡ5.0g������Ʒ�������¶�ʹ��ֽ⣬�ֽ���̵��������±����ش��������⣺
| �¶ȷ�Χ/�� | ��������/g |
| 258��680 | 3.20 |
| 680��1000 | 1.60 |
| 1000���� | 1.44 |
�ٸ��¶���1000�����ϣ�
��ͭԭ�ӵ���λ����4��
���� ��1������X�Ľṹ��ʽ�ж��侧�����ͣ�
��2��Ԫ�صķǽ�����Խǿ���縺��Խǿ����ͬ����������ҵ縺������ͬһ����Ԫ�ش��ϵ��µ縺����С��
��3���ɽṹ��ʽ��֪X�����д�����λ�����ۼ����ݴ˽�ɣ�
��4������������Ĵ��ڣ�����ˮ���Լ�С���ݴ˽�ɣ�
��5������ͼ��֪��ֻ����2��ԭ�ӣ���ΪCu��O���仯ѧʽΪCu2O����������ͭ��������������ʵ����������Cu2O���������ɹ���������ж��¶ȣ�
����ͼ��֪Cu����λ��Ϊ4��
��� �⣺��1����������X�Ľṹ��ʽ�жϣ�X������ˮ���������л��ܼ�����XΪ���Ӿ��壬
�ʴ�Ϊ�����Ӿ��壻
��2��������X�б���C���ʻ�C��sp2�ӻ�����sp3�ӻ���ʽ��ԭ����O��N��C������Nԭ��2p������������һ�����ܸ���O����һ�������ɴ�С˳��Ϊ��N��O��C��
�ʴ�Ϊ��N��O��C��
��3���ɽṹ��ʽ��֪X�����д�����λ�����ۼ���
�ʴ�Ϊ��be��
��4������M���γɷ�����������������ܽ�ȼ�С��
�ʴ�Ϊ��M���γɷ����������ʹ�ܽ�ȼ�С��
��5������ͼ��֪��ֻ����2��ԭ�ӣ���ΪCu��O���þ�����Oԭ����Ϊ1+8��$\frac{1}{8}$=2��Cuԭ����Ϊ4���仯ѧʽΪCu2O������ͭ�����ʵ���=$\frac{5.00g}{250g/mol}$=0.02mol��������ͭ������=0.01mol��144g/mol=1.44g�����Լ����¶���1000�����ϣ��ʴ�Ϊ��1000�����ϣ�
����ͼ��֪Cu����λ��Ϊ4���ʴ�Ϊ��4��
���� ���⿼���Ϊ�ۺϣ��漰������жϡ��縺�ԱȽϡ��������ѧ���������֪ʶ����Ŀ�Ѷ��еȣ�ע�����վ�̯���ھ��������е�Ӧ�ã�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ������������������Ӧ��������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| ��ѧʽ | ���볣�� |
| HF | Ki=3.5��10-4 |
| H2CO3 | Ki1=4.4��10-7 |
| Ki2=4.7��10-11 | |
| HClO | Ki=3.2��10-8 |
| A�� | ͬ��ͬŨ���£���Һ��pH��NaF��NaClO��Na2CO3 | |
| B�� | ���H+��������ClO-��HCO3-��F- | |
| C�� | ����������Һ��ͨ����������̼�����ӷ���ʽ��ClO-+CO2+H2O�THCO3-+HClO | |
| D�� | ̼������Һ�м����������������ӷ���ʽ��CO32-+2HF�T2F-+H2O+CO2�� |

��֪��
�����ǽϻ��ý������ڿ������ױ�������
������������������ˮ�����
��
| �𡡡��� | �۵㣨�棩 | �е㣨�棩 |
| �� | 841 | 1 487 |
| �� | 920 | 3 470 |
��2������2Ҫ��HCl����ķ�Χ�ڼ��ȵ�ԭ���Ƿ�ֹLaCl3ˮ�⣮
��3����������β��Ҫ�ü�Һ���գ���д����Ӧ�����ӷ���ʽCl2+2OH-=Cl-+ClO-+H2O��
��4����ա����¹����еķ�Ӧ����ʽΪ3Ca+2LaF3$\frac{\underline{\;���\;}}{����}$3CaF2+2La��
��5�����羫�ƹ������¶ȿ��Ʒ�Χ1487��3470�森
��6�����������Ƶõ�������Ȼ���м������ĸƣ�ij���β�Ʒ69.709g������������0.209g���ò�Ʒ�ĵȼ�Ϊ��������������Ʒ�ȼ������ż�����99.8%����������99.7%����ѧ����99.5%��
| A�� | �����Ը��������Һ�����������ϩ | |
| B�� | ��BaCl2��ȥNaOH ��Һ�л��е�����Na2SO4 | |
| C�� | ��KSCN��Һ������Һ�к��е�Fe2+ | |
| D�� | ���ܽ⡢���˵ķ�������CaCl2��NaCl�������� |
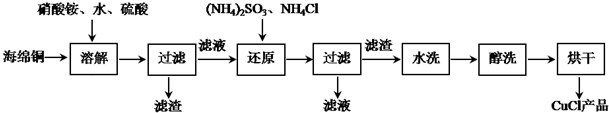
 �ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ��Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺
�ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ��Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺ ��
��
 ��
��

 ��
��