��Ŀ����
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
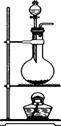
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£��ٽ���ʽ�ζ���������ˮϴ�����ô�����Һ��ϴ����ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶ȵ�λ�ã�����ƿ������ˮϴ�����ô�����Һ��ϴ��ƿ2��3�Σ��Ӽ�ʽ�ζ����з���25.00mL������Һ����ƿ�У�
�ڽ���ʽ�ζ���������ˮϴ��������������ע��0.1000mol/L�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶Ȼ�0���̶����µ�ijһλ�ã�
������ƿ�е����̪��ָʾ�������еζ����ζ�����Һ�պñ�ɫ�������������ΪV1mL��
���ظ����Ϲ������Σ�����������������ֱ�ΪV2mL��V3mL��
�Իش��������⣺
��1����ƿ�е���Һ��
��2���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�
A���ζ�����Һ��ı仯 ��B����ƿ����Һ��ɫ�ı仯
��3����С���ڲ�����еĴ�����
��4�������ȱ�ٵIJ�����
��5����ͼ����ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ
��6�������������ݣ�
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 25.00 | 0.00 | 20.30 |
| �ڶ��� | 25.00 | 0.00 | 20.20 |
| ������ | 25.00 | 4.00 | 24.10 |
���㣺�к͵ζ�
ר�⣺ʵ����
��������1�����ݵζ��յ㣬��ƿ�е���Һ�Ӻ�ɫ��Ϊ��ɫʱ���Ұ�����ڲ���ɫ��Ӧֹͣ�ζ���
��2�����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
��3�����ݴ�����Һ��ϴ��ƿ����ʹ�Ĵ���Һ�����ʵ������ࣻ����c�����⣩=
������
��4��������ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ��
��5�����ݵζ��ܵľ�ȷ��Ϊ0.01mL
��6������c�����⣩=
���㣬V�����������ε�ƽ��ֵ��
��2�����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
��3�����ݴ�����Һ��ϴ��ƿ����ʹ�Ĵ���Һ�����ʵ������ࣻ����c�����⣩=
| c(��)��V(��) |
| V(����) |
��4��������ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ��
��5�����ݵζ��ܵľ�ȷ��Ϊ0.01mL
��6������c�����⣩=
| c(��)��V(��) |
| V(����) |
���
�⣺��1����ζ��յ�ʱ����ƿ�е���Һ�Ӻ�ɫ��Ϊ��ɫʱ���Ұ�����ڲ���ɫ��ֹͣ�ζ����ʴ�Ϊ���죻�ޣ�
��2�����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯���ʴ�Ϊ��B��
��3�����ݴ�����Һ��ϴ��ƿ����ʹ�Ĵ���Һ�����ʵ������࣬���V������ƫ����c�����⣩=
����֪c������ƫ��
�ʴ�Ϊ��������Һ��ϴ��ƿ��ƫ�ߣ�
��4��������ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ��û����ϴ���±�ҺŨ�Ƚ��ͣ�����V������ƫ����c�����⣩=
����֪c������ƫ�ʴ�Ϊ������ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ��ƫ�ߣ�
��5���ζ����е�Һ�����Ϊ22.60mL���ʴ�Ϊ��22.60��
��6��V�������T[��20.30+20.20+��24.10-4.00��]mL��3�T20.20mL
C�����⣩�TC��������V��������V�����⣩�T0.1000mol?L��20.20mL/25.00mL�T0.0808mol?L-1��
�ʴ�Ϊ��0.0808��
��2�����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯���ʴ�Ϊ��B��
��3�����ݴ�����Һ��ϴ��ƿ����ʹ�Ĵ���Һ�����ʵ������࣬���V������ƫ����c�����⣩=
| c(��)��V(��) |
| V(����) |
�ʴ�Ϊ��������Һ��ϴ��ƿ��ƫ�ߣ�
��4��������ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ��û����ϴ���±�ҺŨ�Ƚ��ͣ�����V������ƫ����c�����⣩=
| c(��)��V(��) |
| V(����) |
��5���ζ����е�Һ�����Ϊ22.60mL���ʴ�Ϊ��22.60��
��6��V�������T[��20.30+20.20+��24.10-4.00��]mL��3�T20.20mL
C�����⣩�TC��������V��������V�����⣩�T0.1000mol?L��20.20mL/25.00mL�T0.0808mol?L-1��
�ʴ�Ϊ��0.0808��
������������Ҫ�������к͵ζ������Լ�ע������ѶȲ���Ӧע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��������������������ά�IJ����ǣ�������
| A��ͭ�Ͻ� | B���մ� |
| C������ϩ | D���������� |
��ɫ��ѧ�ĺ����Ƿ�Ӧ���̵���ɫ������Ҫ��ԭ�������е�����ԭ����ȫ��������ȫ��ת�������IJ�Ʒ�У����й��̲�������һ˼����ǣ�������
A��������CO�ϳɼ״���2H2+CO
| ||
B��ϩ����ˮú��������Ӧ��RCH=CH2+CO+H2
| ||
| C��CH4��Cl2��Ӧ��ȡCCl4 | ||
| D������ϩ�ϳɾ�����ϩ |
����ʱ����Ũ�Ⱥ�����ֱ�ΪC1��V1��NaOH��Һ��C2��V2��CH3COOH��Һ���ϣ����й��ڸû����Һ������������ǣ�������
| A����pH��7ʱ����һ����C1V1=C2V2 |
| B�����κ�����¶���c��Na+��+c��H+��=c��CH3COO-��+c��OH-�� |
| C����pH=7ʱ����V1=V2����һ����C2��C1 |
| D����V1=V2��C1=C2����C��CH3COO-��+C��CH3COOH��=C��Na+�� |


 ����Ҫԭ�ϣ��ϳ�ҽҩ�м��壺
����Ҫԭ�ϣ��ϳ�ҽҩ�м��壺 ����ԭ����ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH
����ԭ����ѡ�����ϳ�·������ͼʾ�����£�CH3CH2OH