��Ŀ����
1���±���Ԫ�����ڱ���һ���֣���Ա��еĢ�-����Ԫ�أ���д���пհף�| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A |
| 2 | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� |
��2��Ԫ�آٵķǽ����Ա�Ԫ�آ�ǿ����������֤���ý��۵���ʵ�ǣ�̼������Աȹ��������ǿ��
��3��Ԫ�آڵ���̬�⻯�����ĵ��ʷ����û���Ӧ�ķ���ʽΪ2NH3+Cl2=N2+6HCl��
��4��Ԫ�آۺ͢��γɵĻ������п�������������Ư������Na2O2���ѧʽ����
��5��Ԫ�آۡ��ݺ͢��γɵĻ����ﳣ���ڹ�ҵ��ˮ�Ĵ���������Ҫ����Ϊ������ˮ�����ɽ�����������ԣ��ɾ���ˮ��
���� ��Ԫ�������ڱ���λ�ÿ�֪������C������N������O������Na������Al������Si������S������Cl��
��1��C�γ��л��������ࣻ
��2��̼������Աȹ��������ǿ����˵��C��Si�ķǽ�����ǿ��
��3��������������Ӧ���ɵ�����HCl��
��4��Ԫ�آۺ͢��γɵĻ������п�������������Ư�����ǹ������ƣ�
��5��Ԫ�آۡ��ݺ͢��γɵĻ�����Ϊ�������������ڹ�ҵ��ˮ�Ĵ�������������ˮ�����ɽ����йأ�
��� �⣺��Ԫ�������ڱ���λ�ÿ�֪������C������N������O������Na������Al������Si������S������Cl��
��1������Ԫ���У��γɻ���������������C���ʴ�Ϊ��C��
��2��Ԫ�آٵķǽ����Ա�Ԫ�آ�ǿ����������֤���ý��۵���ʵ��̼������Աȹ��������ǿ���ʴ�Ϊ��̼������Աȹ��������ǿ��
��3��������������Ӧ���ɵ�����HCl����ӦΪ2NH3+Cl2=N2+6HCl���ʴ�Ϊ��2NH3+Cl2=N2+6HCl��
��4��Ԫ�آۺ͢��γɵĻ������п�������������Ư�����ǹ������ƣ��仯ѧʽΪNa2O2���ʴ�Ϊ��Na2O2��
��5��Ԫ�آۡ��ݺ͢��γɵĻ�����Ϊ�������������ڹ�ҵ��ˮ�Ĵ���������Ҫ����Ϊ������ˮ�����ɽ�����������ԣ��ɾ���ˮ��
�ʴ�Ϊ��������ˮ�����ɽ�����������ԣ��ɾ���ˮ��
���� ���⿼��λ�á��ṹ�����ʣ�Ϊ��Ƶ���㣬����Ԫ�ص�λ�á�Ԫ�ص����ʡ�Ԫ�ػ�����֪ʶΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��Ԫ�ػ����P��ط�Ӧԭ����Ӧ�ã���Ŀ�ѶȲ���

| A�� | Ϊ����±�����е�±ԭ�ӣ��ȼ���NaOH��Һ���ȣ��ټ���AgNO3��Һ�۲���ɫ�仯 | |
| B�� | Ϊ��ȡ������������ϡH2SO4���Ҵ��������ϼ��ȣ��ų�������ͨ��ʢ��NaOH��Һ���Թ��� | |
| C�� | Ϊ����ȩ���Ĵ��ڣ������Թ��м���2mL2%��NaOH��Һ������CuSO4��Һ���ټ���ȩ����� | |
| D�� | Ϊ��ȡ��ϩ����ϡH2SO4���Ҵ���ϼ��� |
| A�� | �ı䷴Ӧ������ | B�� | ����ѹǿ | C�� | ������� | D�� | �����¶� |
| A�� | BCl3 | B�� | NCl3 | C�� | H2S | D�� | BeCl2 |
| ������ | K+��Na+��NH4+��Fe2+��Ba2+��Cu2+ |
| ������ | OH-��I-��NO3-��AlO2-��HCO3-��HSO4- |
��A�еĻ�ѧ������Ϊ���Ӽ������ۼ�������Ӽ����������ۼ�������
��A��B��Һ��Ϻ���ȳ����ԣ��÷�Ӧ�����ӷ���ʽΪH++SO42-+NH4++Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$BaSO4��+NH3��+2H2O��
��2����A��ˮ��ҺΪdz��ɫ��B����ɫ��Ӧ�ʻ�ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ��Ϻ������Ա仯����
��A�Ļ�ѧʽΪFeI2��
�ھ�����������������Һ��Ƶ�ԭ����������֣���������������
�����I-��������I2ʹ��Һ�ʻ�ɫ����I-��Fe2+��������ʹ��Һ�ʻ�ɫ��
������һ������֤��������Һ��Ƶ�ԭ��ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������������������ɣ���
������������������Һ���ԭ����������Ƴ�ԭ��أ���������a����b����b���ĵ缫��ӦʽΪNO3-+4H++3e-�TNO��+2H2O��
| A | B | C | D | |
| ���� | 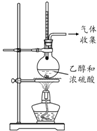 |  | 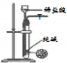 | 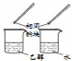 |
| Ŀ�� | �����Ҵ�����ȥ��Ӧ��ȡ��ϩ | ����NH4Cl������Һ�Ʊ�NH4Cl���� | ��ȡ����������CO2���� | �Ƚ��Ҵ����ǻ���ԭ�Ӻ�ˮ��������ԭ�ӵĻ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
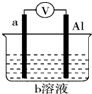 ��ͼ��ijͬѧ��Ƶ�һ������ԭ���װ�ã��ش��������⣮
��ͼ��ijͬѧ��Ƶ�һ������ԭ���װ�ã��ش��������⣮