��Ŀ����
��ѧ��һ����ʵ��Ϊ���Ŀ�ѧ����ѧʵ����ѧϰ̽���������ʵĻ�������֮һ��
��1����ѧʵ���У�������Һ���Լ������ữ�������ữ�����Ĵ�ʩ����ȷ���� ��
A�����Լ���SO32-������HNO3�ữ��BaCl2��Һ
B������FeCl2��Һʱͨ��������HNO3�ữ����С��ˮ��̶�
C������ij��Һ���Ƿ�Cl-����HNO3�ữ��AgNO3��Һ
D�����Ը��������Һ�����������ữ
��2�������й�˵������ȷ���� ��
����pH��ֽ�����ˮ��pHΪ3.5
��������������NaOH����
�ۼ���ŨNaOH��Һ�����Ȳ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��ԭ��Һ��һ������NH4+
������Һ�м�������˫��ˮ���ټӼ���KSCN��Һ����Һ��죬��ԭ��Һ��һ������Fe2+
��ʵ��ʱ����������������ָ����������Ѫʱ���������Ȼ�����ҺͿĨֹѪ��
��1����ѧʵ���У�������Һ���Լ������ữ�������ữ�����Ĵ�ʩ����ȷ����
A�����Լ���SO32-������HNO3�ữ��BaCl2��Һ
B������FeCl2��Һʱͨ��������HNO3�ữ����С��ˮ��̶�
C������ij��Һ���Ƿ�Cl-����HNO3�ữ��AgNO3��Һ
D�����Ը��������Һ�����������ữ
��2�������й�˵������ȷ����
����pH��ֽ�����ˮ��pHΪ3.5
��������������NaOH����
�ۼ���ŨNaOH��Һ�����Ȳ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��ԭ��Һ��һ������NH4+
������Һ�м�������˫��ˮ���ټӼ���KSCN��Һ����Һ��죬��ԭ��Һ��һ������Fe2+
��ʵ��ʱ����������������ָ����������Ѫʱ���������Ȼ�����ҺͿĨֹѪ��
���㣺��ѧʵ�鷽��������,��ѧʵ�鰲ȫ���¹ʴ���
ר�⣺ʵ��������
��������1���ữʱ��ע��������������Һ���ܷ�����Ӧ��������Һ���ʣ�
��2���ٹ㷺pH��ֽ��ȷ��1��
���������ƿ����մ��ж������跴Ӧ��
��ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ������
�ܼ����Լ�˳�����
�ݽ������������Һ�����۳���
��2���ٹ㷺pH��ֽ��ȷ��1��
���������ƿ����մ��ж������跴Ӧ��
��ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ������
�ܼ����Լ�˳�����
�ݽ������������Һ�����۳���
���
�⣺��1��A�����Լ���SO32-������HNO3�ữ��BaCl2��Һ�������������ǿ�����ԣ�������SO32-������֤��SO32-�Ĵ��ڣ���A����
B������FeCl2��Һʱ�����ܼ�����HNO3�ữ����������FeCl2��������Һ���ʣ���B����
C������ij��Һ���Ƿ�Cl-����HNO3�ữ��AgNO3��Һ���ɼ��������ӣ���C��ȷ��
D�����Ը��������Һ����ǿ�����ԣ����������ᣬ�����������ữ��Ӧ�����ᣬ��D����
�ʴ�Ϊ��C��
��2���ٹ㷺pH��ֽ��ȷ��1���ʴ���
���������ƿ����մ��ж������跴Ӧ���ɿڵ�������ը�ѣ��ʴ���
��ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ�����������ڼ���笠����ӣ�����ȷ��
�ܼ����Լ�˳�����Ӧ�ȼ�KSCN��Һ���ʴ���
�ݽ������������Һ�����۳��������Ȼ���ֹѪ������ȷ��
�ʴ�Ϊ���ۢݣ�
B������FeCl2��Һʱ�����ܼ�����HNO3�ữ����������FeCl2��������Һ���ʣ���B����
C������ij��Һ���Ƿ�Cl-����HNO3�ữ��AgNO3��Һ���ɼ��������ӣ���C��ȷ��
D�����Ը��������Һ����ǿ�����ԣ����������ᣬ�����������ữ��Ӧ�����ᣬ��D����
�ʴ�Ϊ��C��
��2���ٹ㷺pH��ֽ��ȷ��1���ʴ���
���������ƿ����մ��ж������跴Ӧ���ɿڵ�������ը�ѣ��ʴ���
��ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ�����������ڼ���笠����ӣ�����ȷ��
�ܼ����Լ�˳�����Ӧ�ȼ�KSCN��Һ���ʴ���
�ݽ������������Һ�����۳��������Ȼ���ֹѪ������ȷ��
�ʴ�Ϊ���ۢݣ�
���������⿼�黯ѧʵ�鷽�������ۣ��漰��Һ�����ơ����ʵļ����֪ʶ���ɽ�𣬲�����ѧ���ķ���������ʵ�����������������Ŀ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
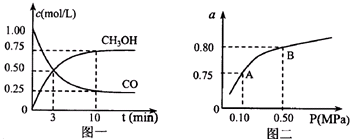
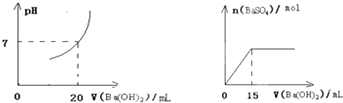
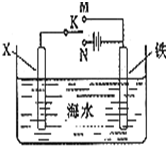 ��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���