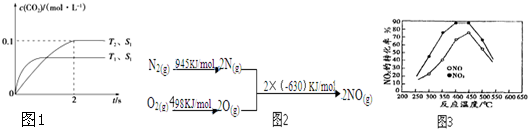
��Ŀ����
ij����Ҫ��H2SO4��HF������ᣩ�Ļ����Һ��������ϡ��Ԫ�ص���ȡҺ��Ҫ�����ȡҺ��H2SO4��HF�����ʵ���Ũ�ȸ�Ϊ3.16mol/L��9.00mol/L������500L������Һ�����ⶨ���У�H2SO4��HF�����ʵ���Ũ�ȸ�2.15mol/L��12.6mol/L����Ҫ�ô�500L������Һ����������ȡҺ���ٴ˻�����Һ��ϡ���ɵõ� L�Ϸ�����Ҫ�����ȡҺ�����ڴ�500L�Ļ�����Һ��Ӧ���� L�ܶ�Ϊ1.84g/ml��Ũ��Ϊ98%��Ũ���ᣬ�ŷ�����ȡҺ�Ļ���Ҫ��
���㣺��Һ������
ר�⣺ʵ����,������
����������ϡ�ͺ���Һ���ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ��HF�����ʵ������䣬�ݴ˼���ϡ�ͺ���Һ�������
�ڷ�����ȡҺҪ��Ӧ��֤���ᡢ������Ũ�ȷֱ�Ϊ3.16mol/L��9.00mol/L������������Ũ�ȿ�֪����Ũ���ᣬ�ټ�ˮϡ�ͺ���Һ������������Ũ��������Ϊx������H2SO4���ʵ����غ��з��̼������ҪŨ�����������ɣ�
�ڷ�����ȡҺҪ��Ӧ��֤���ᡢ������Ũ�ȷֱ�Ϊ3.16mol/L��9.00mol/L������������Ũ�ȿ�֪����Ũ���ᣬ�ټ�ˮϡ�ͺ���Һ������������Ũ��������Ϊx������H2SO4���ʵ����غ��з��̼������ҪŨ�����������ɣ�
���
�⣺����ϡ�ͺ���Һ���ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ��HF�����ʵ������䣬���У�9.00mol?L-1��V=12.6mol?L-1��500L����ã�V=700L��
�ʴ�Ϊ��700��
�ڷ�����ȡҺҪ��Ӧ��֤���ᡢ������Ũ�ȷֱ�Ϊ3.16mol/L��9.00mol/L�����ݷ������Ũ�ȿ�֪��֪����Ũ���ᣬ�ټ�ˮϡ�ͺ���Һ�����Ϊ700L��
�����Ũ��������Ϊx������H2SO4���ʵ����غ㣬���У�
+500L��2.15mol?L-1=700L��3.16��mol?L-1�����x=6.18��104 mL=61.8L��
�ʴ�Ϊ��61.8��
�ʴ�Ϊ��700��
�ڷ�����ȡҺҪ��Ӧ��֤���ᡢ������Ũ�ȷֱ�Ϊ3.16mol/L��9.00mol/L�����ݷ������Ũ�ȿ�֪��֪����Ũ���ᣬ�ټ�ˮϡ�ͺ���Һ�����Ϊ700L��
�����Ũ��������Ϊx������H2SO4���ʵ����غ㣬���У�
| x��1.84glcm3��98% |
| 98g/mol |
�ʴ�Ϊ��61.8��
���������⿼������Һ�����ơ����ʵ���Ũ�ȵļ��㣬��Ŀ�Ѷ��еȣ�ע������һ�����ʵ���Ũ�ȵ���Һ�����Ʒ�������ȷ���ʵ���Ũ�ȵĸ�����㷽����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ